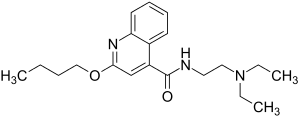
Dibucaine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cinchocaine | |||||||||||||||||||||
| other names |
2-butoxy- N - (2-diethylaminoethyl) -quinoline-4-carbamide ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 20 H 29 N 3 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 343.46 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dibucaine ( cinchocaine ) is a local anesthetic of the amide type. As one of the first of its kind, it was synthesized around 1930. In 1931 the active ingredient was patented by CIBA AG . Today, dibucain is particularly important in laboratory diagnostics and in the treatment of inflammatory diseases of the anus. It has a slow onset of action and a duration of approx. 1.5–2 hours. As a surface anesthetic, it is 100 times more effective than cocaine . The toxicity is also quite great; it is 15-20 times the toxicity of the relatively less toxic procaine .
Pharmacokinetics
Dibucaine can be absorbed through the skin. The active ingredient is broken down in the liver and excreted in the urine.
application areas
- As a substrate for pseudocholinesterase , dibucaine is used in the laboratory diagnostics of atypical forms of this enzyme . In the dibucaine test introduced in 1957 by W. Kalow and K. Genest, the so-called dibucaine number provides information about the activity of pseudocholinesterase after the addition of dibucaine.
- In combination with other active ingredients (for example in Otobacid : dexamethasone , cinchocaine hydrochloride and butane-1,3-diol ), dibucaine is used in various areas of medicine - for example in ear, nose and throat medicine - as a locally acting anesthetic .
Trade names
DoloPosterine (D)
Decatylen Neo (CH), Faktu (D, CH), Locaseptil (CH), Otobacid (D), Scheriproct (A, CH)
Individual evidence
- ↑ a b Dibucaine data sheet at Sigma-Aldrich , accessed on November 6, 2016 ( PDF ).
- ↑ Entry on dibucain in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry on cinchocaine. In: Römpp Online . Georg Thieme Verlag, accessed on January 24, 2019.
- ↑ Eberhard Schröder, Clemens Rufer, Ralph Schmiechen, Arzneimittelchemie I, Georg Thieme Verlag 1976 ISBN 3-13-520601-7
- ↑ Michael Heck, Michael Fresenius: Repetitorium Anaesthesiologie. Preparation for the anesthesiological specialist examination and the European diploma in anesthesiology. 3rd, completely revised edition. Springer, Berlin / Heidelberg / New York et al. 2001, ISBN 3-540-67331-8 , p. 804.
- ↑ Landessozialgericht Berlin-Brandenburg: L 7 KA 11/10 KL ER . Retrieved November 20, 2010.
- ↑ Red List online, as of October 2009.
- ↑ AM comp. d. Switzerland, as of October 2009.
- ↑ AGES-PharmMed, as of October 2009.