antibody

Antibodies ( immunoglobulins , in international parlance also immunoglobulin , outdated gamma globulin ) are proteins from the class of globulins that are formed (synthesized) in vertebrates as a reaction product of special body cells (plasma cells) to certain substances (substances called antigens ). Antibodies serve the immune system . Antibodies are made by a class of white blood cells called plasma cells in response to a response from B lymphocytes .
The antigens are almost exclusively macromolecules or molecules bound to particles , for example lipopolysaccharides on the surface of bacteria. A certain antigen usually induces the formation of only a few, very specific, matching antibodies, which mostly only recognize this foreign substance via specific, non- covalent bonds (one has, for example, with the Smallpox vaccination made use of: The antibodies formed by the body against the harmless cowpox also recognize smallpox viruses that are pathogenic for humans). The specific binding of antibodies to the antigens forms an essential part of the defense against the invading foreign substances. In the case of pathogens as foreign substances, the formation and binding of antibodies can lead to immunity . Antibodies are central components of the immune system of higher vertebrates.
As was first described in 1948 by the Swedish immunologist Astrid Fagraeus, antibodies are secreted by a class of white blood cells ( leukocytes ) , which are known as effector cells or plasma cells and represent differentiated B lymphocytes. They occur in the blood and in the extracellular fluid of the tissue and usually do not “recognize” the entire structure of the antigen, but only part of it, the so-called antigenic determinant (the epitope ). The specific antigen binding site of the antibody is called the paratope . Upon contact with the antigen, the antibodies generate what is known as the humoral immune response ( humoral defense ).
Structure of Antibodies
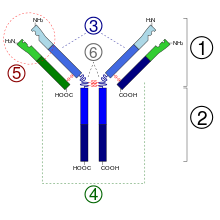
1. Fab section
2. Fc section
3. heavy chains
4. light chains
5. antigen binding site ( paratope )
6. hinge region
(*) -SS disulfide bridge
Since some amino acid residues have sugar chains, antibodies are part of the glycoproteins . Each antibody is composed of two identical heavy chains (engl. Heavy chains , H) and two identical light chains (engl. Light chains , L) connected by covalent disulfide bridges between the chains (so-called inter-chain disulfides ) to a Wye-shaped structure together are linked. The light chains (also: light chains ) consist of a variable and a constant domain . Are referred to it as V L and C L . The heavy chains (also: heavy chains ), however, each have one variable and three (IgG, IgA) or four (IgM, IgE) constant domains. These are referred to analogously as V H and C H 1, C H 2, C H 3.
The variable domains of a light and a heavy chain together form the antigen binding site. The constant domain C H 2 consists u. a. also from a carbohydrate chain, which forms a binding site for the complement system. The constant domain C H 3 is the Fc receptor binding site for opsonization . The variable domains in turn form different characteristic paratopes which together form an idiotype .
The two light chains are κ depending on the organism and immunoglobulin subclass either the type or λ and, together with the above the hinge region (engl. Hinge region , including: hinge region ) lying portion of the heavy chain antigen-binding fragment Fab . (Engl antigen-binding fragment ), which can be cleaved enzymatically with the help of papain from the underlying crystallizable fragment Fc (English crystallizable fragment ). The organism achieves the extraordinary variability of the antigen binding sites (English Complementarity Determining Region , CDR) by means of V (D) J recombination .
Papain cleaves above the inter-chain disulfide bridges of the two heavy chains. This gives two Fab fragments and a complete Fc fragment . In contrast, pepsin cleaves below the disulfide bridges. The hinge region is retained between the two Fab fragments. This fragment is then called F (ab) 2 . Pepsin and plasmin also cleave the Fc fragment between the second and third domains of the constant part of the heavy chain.
Antigen - Antibody Binding
Antibodies bind with their A (necessary) B (indungs) region to “their” paratope relatively specifically, analogous to the key-lock principle . It is not uncommon, however, that, metaphorically speaking, a second or third key exists that fits into the antibody “lock” due to the (coincidentally) similar or identical configuration of the epitope . With a very low probability, this can also be an endogenous structure. An approach to explaining autoimmune diseases is based on this phenomenon .
The bond between epitope and immunoglobulin is non-covalent and is subject to the law of mass action . Effective agglutination , that is, clumping through the formation of large complexes, is therefore only possible with approximately the same number of epitopes and binding sites. With large deviations upwards or downwards, the complexes remain in solution; Nevertheless, the antigens are usually neutralized. Neutralizing antibodies effective against multiple virus strains are referred to as broadly neutralizing antibodies , e.g. B. Broadly Neutralizing Anti-HIV Antibodies .
Antibodies as B-cell receptors
Membrane-bound antibodies (called B-cell receptors ( BCR )) can activate B-cells when they are cross-linked by antigens. The B cell then takes up the immune complex by endocytosis , digests the antigen proteolytically and presents fragments of it on its cell surface via MHC class II molecules ( peptides with 13-18 amino acids ). If the presented fragments are also recognized by a CD4 T cell (T helper cells) (in parallel on other professional antigen presenting cells or on this same B cell) , this T cell stimulates the B cell, which leads to further maturation processes ( somatic hypermutation , class change ) and proliferation to antibody- secreting plasma cells or / and to B memory cells. This maturation processes found within germinal centers ( germinal center ) in the secondary lymphoid organs ( spleen , lymph nodes ) and will be under the concept of germinal center reaction ( germinal center reaction combined).
With the exception of a small part at the carboxyl end of the heavy chain, the B-cell receptor is identical to the antibody of the respective B-cell. The B-cell receptor has a hydrophobic sequence anchored in the cell membrane, whereas the antibody has a hydrophilic sequence that causes its secretion. The two forms arise through alternative RNA processing .
Mode of action of secreted antibodies
Secreted antibodies work through different mechanisms:
- The simplest is the neutralization of antigens, measurable in a neutralization test . Because the antibody binds the antigen, it is blocked and, for example, can no longer develop its toxic effect, or other interactions of the antigen with body cells are prevented, e.g. B. the penetration of bacteria or viruses into cells or tissues.
- Another is opsonization ("making it palatable"), the enveloping of pathogens and foreign particles with antibodies to mark for the immune system. For example, when an antibody binds to an antigen that is on the surface of a bacterium, it simultaneously marks the bacterium, because the constant region of the antibody that has bound to its antigen is recognized by phagocytes , which act as scavenger cells ingesting the bacterium and digest.
- A third mode of action is that antibodies activate the complement system. These are IgM (as a monomer) and IgG oligomers formed even after binding an IgG, with increased activation by IgG hexamers . This leads to a perforation of the marked cell.
- Antibodies that bind to the body's own cells can activate NK cells , which then kill these cells. This process is also known as antibody-dependent cellular cytotoxicity (ADCC).
- Because an antibody has two antigen binding sites, agglutination can occur.
- In adenoviruses one was intracellular antibody-mediated degradation described.
Different classes of antibodies
In most vertebrates there are five different classes (isotypes) of immunoglobulins, which are classified according to their different gene segments for the constant parts of the heavy chain. In addition, there are some classes that can only be found in individual groups of animals. The different isotypes occur in different compartments of the body and have different tasks.
Immunoglobulin A
Immunoglobulin A (IgA) is secreted on all mucous membranes of the respiratory tract , the eyes , the gastrointestinal tract, the urogenital tract and via special glands around the nipple of mothers and protects there against pathogens (including the newborn). Secreted IgA occurs in the form of homodimers ; the two parts are linked by the joining peptide .
Immunoglobulin D
Immunoglobulin D (IgD) is presented in membrane form on mature, naive (antigen-inexperienced) B-cells by alternative splicing of IgM / IgD pre-mRNA together with IgM as a B-cell receptor (BCR) . IgD are 170–200 k Da large monomers and only present in secreted form in small amounts in blood and lymph (less than 1%). IgD is not secreted by plasma cells and is rapidly broken down in free form. It acts as an antigen receptor in the antigen-stimulated multiplication and in the differentiation of B cells.
Immunoglobulin E.
Immunoglobulin E (IgE) provides protection against parasites such as B. pathogenic worms, and is implicated in allergies . IgE are 190 kDa monomers. It is bound with high affinity by Fc receptors on mast cells as well as basophils and eosinophils . For this reason, almost all IgE is membrane-bound, it is practically not present in the blood. When it comes into contact with the antigen, it is cross-linked , which leads to the release of histamine , granzymes, etc. by the mast cells (mast cell degranulation - this is where allergy drugs that “stabilize” the mast cells take effect) and granulocytes (immediate allergic reaction, anaphylactic reaction).
Immunoglobulin G
Immunoglobulins G (IgG) are 150 kDa monomers with a sedimentation coefficient of 7S. This class of antibodies is only formed in a late defense phase, around 3 weeks after infection, and is retained for a long time. The evidence indicates a passed infection or vaccination. The immunizing function is based on two antigen-bound IgGs that activate the complement system. The Fc receptor mediates phagocytosis .
An example is anti-measles IgG, antibodies of the IgG class directed against the measles virus, as a sign of current or previous infection or vaccination . The Rh factor- D antibodies are also of this type, which can lead to complications during pregnancy, since immunoglobulin G crosses the placenta. Even without complications, IgG in the maternal blood is actively transported through the placenta (barrier) into the fetus and provides initial protection against infections after birth.
IgGs are divided into the following subclasses: IgG1-IgG4 (human) or IgG1, IgG2, IgG2b and IgG3 (mouse).
Diseases with a congenital or acquired antibody deficiency often involve IgG. If the body forms antibodies, so-called autoantibodies , against its own body components, one speaks of an autoimmune disease .
Pharmacokinetics
Immunoglobulins are immediately and completely bioavailable in the recipient's bloodstream after intravenous administration. They distribute relatively quickly between plasma and extravascular fluid; after about three to five days, an equilibrium between the intra- and extravascular compartments is achieved. The in vivo half-life of IgG in patients with primary antibody deficiency syndrome is 35 days. However, the half-life of IgG can vary from patient to patient, especially in those with primary immune deficiency syndromes. Immunoglobulins and IgG complexes are broken down in the cells of the mononuclear phagocytic system.
pharmacology
Immunoglobulin G has a broad spectrum of antibodies against various infectious pathogens. Opsonization and neutralization of microbes and toxins by specific antibodies have been demonstrated. IgG antibodies are made from plasma from at least 1,000 donors; the subclass distribution corresponds to that of human plasma. Corresponding dosages can raise the lowered IgG serum level to normal. The mechanism of action in areas of application other than substitution therapy has not yet been fully explored, but includes immunomodulatory effects. The finished products are adjusted to a weakly acidic pH. No change in blood pH was measured after administration of high doses of IgG. The osmolality of finished medicinal products is close to the physiological values (285–295 mOsmol / kg).
Preclinical safety data
Immunoglobulins are normal components of the human body. The acute toxicity in animals cannot be determined as the volume to be administered would be above the tolerable limit. Animal studies on chronic toxicity and embryotoxicity are not possible as these are disrupted by the formation of antibodies against human proteins. Clinical experience has not provided any indications of carcinogenic or mutagenic effects. Therefore, experimental studies on animals were not considered necessary.
Immunoglobulin M
Immunoglobulin M (IgM) is the class of antibodies that is formed upon initial contact with antigens and indicates the acute phase of infection of a disease, for example anti-measles IgM, antibodies of the IgM class directed against the measles virus as a sign of a fresh infection . Therefore, a recent increase in IgM is generally an important indicator for an initial infection.
IgM is a pentamer (oligomer) made up of five subunits of 180 kDa each. These subunits are linked by the cysteine-rich , 15 kDa joining peptide (J chain). The molecular mass of the IgM pentamer is 970 kDa, the sedimentation coefficient 19S. Since IgM has 10 binding sites for antigens, these antibodies lead to strong agglutination. The antigen-antibody complex of IgM pentamers activates the classic path of the complement system , and the AB0 blood groups are also recognized by IgM antibodies. IgM accounts for 10% of the total Ig.
Immunoglobulin W
Immunoglobulin W (IgW) was only discovered in a species of shark in 1996 . Because of this, it was originally assumed that it only occurs in cartilaginous fish . In 2003, however, IgW was also detected in lung fish , a class of bony fish . IgW probably has some properties of a hypothetical primal immunoglobulin and is therefore of particular interest for research on the evolution of the immune system.
Immunoglobulin Y
Immunoglobulin Y (IgY) also called Chicken IgG , Egg Yolk IgG or 7S-IgG , is the functional equivalent of IgG in chickens and is similar in structure. It can be found in high concentrations in chicken eggs . For use for bioanalytical purposes in immunassays , IgY offers several advantages over IgG.
Application of antibodies in medicine
Antibodies (antisera) obtained from animals are used as therapeutic agents for a wide variety of purposes. An important example is its use as a passive vaccine .
Intravenous immunoglobulins (IVIG) are approved for substitution treatment in various congenital or acquired disorders of antibody formation (e.g. in chronic lymphocytic leukemia , multiple myeloma or after allogeneic hematopoietic stem cell transplantation ) and for immunomodulation in some autoimmune diseases (e.g. immune thrombocytopenia , guillain Barré syndrome ) and diseases of unknown etiology (e.g. Kawasaki syndrome ). The cross-sectional guidelines of the German Medical Association for therapy with blood components and plasma derivatives also mention off-label use in various indications. Reversible hemolytic reactions are one of the long-known possible rare side effects of IVIG preparations. These are probably triggered by antibodies against blood group antigens (isoagglutinins), which IVIG preparations may contain. In March 2013, the Drug Safety Mail of the German Medical Association (AkdÄ) addressed reports of severe hemolytic reactions after intravenous administration of immunoglobulins.
In addition, specific monoclonal antibodies have recently been used therapeutically in medicine. The main area of application is hematology and oncology , but they are also used in the treatment of autoimmune diseases such as multiple sclerosis , rheumatoid arthritis (RA) or CIDP (chronic inflammatory demyelizing polyneuropathy ), AIDP (acute inflammatory demyelizing polyneuropathy), and MMN ( multifocal motor neuropathy related) used for neuromuscular diseases. These antibodies recognize pro-inflammatory cytokines such as interleukin-1 or lymphotoxin-α . Antibodies against the B-cell surface marker CD20 recognize only naive (antigen-inexperienced) and memory B-cells, but not plasma cells (CD20neg), but this therapy is also relatively successful. Antibodies thus represent a class of drugs that is for the first time able to specifically intervene in inflammatory processes ( biologicals ).
In immunoscintigraphy , antibodies are used to locate certain target structures, for example tumor cells , in the body. For this purpose, a usually very short-lived radionuclide is coupled to the antibody . By scintigraphy or positron emission tomography can then determine where the antibody or its target structure (to the target ) is accurate.
In the past, the constant part of the antibodies was still of murine (from the mouse) origin, which could lead to rejection reactions by the immune system. So-called humanized antibodies have recently been used to circumvent this problem . In addition to the variable region mediating the specificity against human antigens, conventional monoclonal antibodies still contain protein components of the mouse, which the human immune system may reject as foreign. With the help of molecular biological processes, the murine parts of the constant sections are therefore removed and replaced by identical constant parts of human antibodies. The constant sections of the antibodies play no role in the specific binding of the monoclonal antibody. The resulting monoclonal antibody is called a "humanized monoclonal antibody" and is no longer rejected by the human immune system. Humanized antibodies are produced in a culture from hamster ovarian cells, which is why their production is much more complex and therefore more expensive than production in microorganisms.
In radioimmunotherapy , an ionizing radiation source with the shortest possible range is coupled to an antibody. Short -lived beta emitters , more rarely alpha emitters , with a short range in the tissue are used here.
In the development of vaccines against variable pathogens are breitneutralisierende antibody (bnAb) examined, which are effective against multiple strains of a virus, for example breitneutralisierende anti-IAV antibody and breitneutralisierende anti-HIV antibody .
Application of antibodies in biology
The high specificity with which antibodies recognize their antigen is used in biology to make the antigen, in the vast majority of cases a protein, visible. The antibodies are either coupled (labeled) directly with an enzyme (converts a substrate into color or chemiluminescence), with fluorescent dyes or with radioactive isotopes or are detected with a secondary antibody that binds to the first (primary antibody) and is labeled accordingly.
- Abzyme
- Chromatin Immunoprecipitation
- Drugwipe test
- ELISA : Quantification of antigens or antibodies in serum, cell culture supernatants, etc. using enzyme-linked antibodies
- ELISPOT : Detection of antibody- or antigen-secreting cells (plasma cells, cytokine-secreting cells) using enzyme-linked antibodies
- EMSA : When the bound protein is detected using Supershift
- FACS : Quantification of cells using fluorescence-coupled antibodies against antigens on the cell surface, in the cytoplasm or in the cell nucleus
- Immunohistochemistry : Detection of an antigen on a cell surface, in the cytoplasm or in the cell nucleus by means of antibodies on tissue thin sections (cryo or paraffin) and thus indirect detection of cell types, differentiation stages, etc.
- Immunoprecipitation
- Phage display
- pregnancy test
- Western blot
Obtaining antibodies
Monoclonal antibodies
A monoclonal antibody is directed against exactly one specific epitope of an antigen. As described for polyclonal antibody production, animals must first be immunized and then their plasma cells (from the spleen or lymph nodes) must be obtained. Since the plasma cells have lost the ability to divide, they must first fuse with tumor cells. The resulting cell hybrids ( hybridoma technology ) receive from the plasma cells the property of producing and secreting a certain antibody and from the tumor cell the ability to theoretically divide infinitely often in culture and thus theoretically live infinitely long. By multiple isolation (cloning) a strain of cells is obtained, which goes back to a single hybridoma cell and thus to a single plasma cell.
The cell lines obtained in this way can now be expanded infinitely in culture and thus theoretically produce infinitely large amounts of antibodies. Since all cells can be traced back to a single cell, all cells in a culture are identical copies of the same cell. Because of this, all cells also produce a certain, identical antibody, which can be precisely defined in terms of its properties (e.g. binding site on the antigen, strength of the binding, etc.) and which can theoretically be produced in unlimited quantities.
Recombinant antibodies
Recombinant antibodies are produced in vitro , that is, without a test animal. Recombinant antibodies are typically made from gene libraries that are suitable for the production of the antibodies in microorganisms. The correct (= specifically binding antibody) is not selected by the immune system of an animal / human, but by a binding step in the test tube. Recombinant antibodies can be used in a variety of ways because they can easily be changed because their genetic material is known. In this way, their binding strength or stability can be improved, or proteins with other functions can be attached, e.g. B. for the generation of bispecific antibodies or immunotoxins .
Polyclonal antisera
Polyclonal antisera are a mixture of different antibodies directed against various epitopes. First, the antigen against which the antibody is to be directed must be selected and produced. This can be achieved in a number of ways, for example by isolating a protein, synthesizing a peptide in vitro , or producing the protein as a whole recombinantly in bacteria . The protein is then injected into an animal, whose immune system then makes antibodies against the protein. This process is called "immunization". Mice, rats and rabbits, but also goats, sheep and horses are used as antibody producers. The immunization is repeated several times. After a few weeks, the polyclonal antiserum can be removed. This contains various antibodies directed against the antigen which are formed by the immunization and which can differ in the epitope recognized.
pathology
The lack of antibodies is called hypogammaglobulinaemia and their complete absence is called agammaglobulinaemia . Too much antibody is called hypergammaglobulinemia . The diagnosis of a disorder of the antibody is usually caused by the protein electrophoresis of blood serum asked. This may have to be supplemented by immunoelectrophoresis .
Causes a more pronounced Hypo gammaglobulinämie can u. a. be:
- congenital deficiency (the most common are congenital immunoglobulin A deficiency or a deficiency in one of the four subclasses of immunoglobulin G)
- Diseases with disorders of the lymphocytes:
Causes a more pronounced Hyper gammaglobulinämie can u. a. be:
- chronic inflammation
- Diseases with disorders of the lymphocytes :
- malignant lymphomas, e.g. B. plasmacytoma / multiple myeloma or Waldenström's disease
- Cirrhosis of the liver
Modified Antibodies
Various derivatives of antibodies with partially altered properties have been generated by protein design , e.g. B. F (ab) 2 fragments , Fab fragments , scFv fragments , single domain antibodies or microantibodies .
literature
- Stefan Dübel, Frank Breitling, André Frenzel, Thomas Rostock, Andrea LJMarschall, Thomas Schirrmann, Michael Hust: Recombinant Antibodies . Textbook and compendium for study and practice. 2nd Edition. Springer Spectrum, Berlin 2019, ISBN 978-3-662-50275-4 , doi : 10.1007 / 978-3-662-50276-1 .
- Stefan Dübel, Janice M. Reichert (Eds.): Handbook of Therapeutic Antibodies . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2014, ISBN 978-3-527-32937-3 (English).
Web links
Individual evidence
- ↑ Eduardo Padlan: Anatomy of the antibody molecule . In: Mol. Immunol. . 31, No. 3, February 1994, pp. 169-217. doi : 10.1016 / 0161-5890 (94) 90001-9 . PMID 8114766 .
- ↑ New Sculpture Portraying Human Antibody as Protective Angel Installed on Scripps Florida campus . Archived from the original on November 18, 2010. Retrieved December 12, 2008.
- ↑ Protein sculpture inspired by Vitruvian Man . Archived from the original on November 18, 2010. Retrieved December 12, 2008.
- ↑ Biorama.ch: Antigen - Antibody - Binding ( Memento from June 18, 2007 in the Internet Archive )
- ↑ Christoph A. Diebolder et al .: Complement is activated by IgG hexamers assembled at the cell surface . In: Science (New York, NY) . tape 343 , no. 6176 , March 14, 2014, p. 1260–1263 , doi : 10.1126 / science.1248943 , PMID 24626930 , PMC 4250092 (free full text).
- ↑ a b c d Stefan HE Kaufmann: Antibodies and their antigens . In: Sebastian Suerbaum, Gerd-Dieter Burchard, Stefan HE Kaufmann, Thomas F. Schulz (eds.): Medical microbiology and infectious diseases . Springer-Verlag, 2016, ISBN 978-3-662-48678-8 , pp. 52 , doi : 10.1007 / 978-3-662-48678-8_8 .
- ↑ a b c d e f Stefan HE Kaufmann: Antibodies and their antigens . In: Sebastian Suerbaum, Gerd-Dieter Burchard, Stefan HE Kaufmann, Thomas F. Schulz (eds.): Medical microbiology and infectious diseases . Springer-Verlag, 2016, ISBN 978-3-662-48678-8 , pp. 51 , doi : 10.1007 / 978-3-662-48678-8_8 .
- ↑ Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg) (PDF; 264 kB), EMA website, Committee for Medicinal Products for Human Use (CHMP), October 21, 2012.
- ↑ Cross-sectional guidelines (BÄK) for therapy with blood components and plasma derivatives. (PDF; 1.5 MB) 4th, revised and updated edition 2014. Board of the German Medical Association , accessed on July 4, 2016 .
- ↑ Increased reporting rate of severe hemolytic reactions after intravenous administration of immunoglobulins , Bulletin on drug safety (page 15 ff), PEI website, June 2012.
- ↑ Drug Safety Mail 2013-16 , AkdÄ website, March 2013.

