Adenoviridae
| Adenoviridae | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
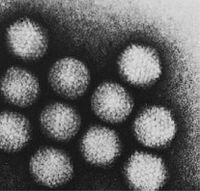
Adenoviridae |
||||||||||||||
| Systematics | ||||||||||||||
|
||||||||||||||
| Taxonomic characteristics | ||||||||||||||
|
||||||||||||||
| Scientific name | ||||||||||||||
| Adenoviridae | ||||||||||||||
| Left | ||||||||||||||
|
The family Adenoviridae (from ancient Greek αδένας 'gland') or adenoviruses (singular: adenovirus ) comprises non-enveloped viruses with a double-stranded, linear DNA as genome . Your capsid has an icosahedral symmetry and has particular, the virus family characterizing construction of so-called Penton - and hexon - capsomeres . Typical antenna-like fiber proteins are anchored to the pentons, giving the virions of the family their “satellite-like” appearance. The family currently includes 47 human and animal pathogenic virus species with numerous subtypes in mammals , birds , reptiles and fish . In humans, the human adenoviruses mainly cause diseases of the respiratory tract .
The first adenoviruses were isolated in 1953 by a working group led by Wallace P. Rowe and Robert J. Huebner from tonsils and other adenoid glandular tissue of children and propagated in a cell culture . These viruses, found among other things in colds, were also referred to as adenoidal pharyngeal conjunctival viruses ( APC viruses ) and adenoid degeneration agents ( AD agents , in German AD agents ), as they cause necrotic changes in adenoid tissue. The Adenoviridae play an important role in the history of molecular biology , as a fundamental process of gene expression , known as splicing , was discovered in them in 1977 . In genetic engineering and virological research, adenoviruses are of certain importance for the introduction of DNA as a viral vector into cells and organisms.
morphology
The non-enveloped capsids of the Adenoviridae are between 70 and 90 nm in diameter and consist of 252 capsomeres . There are two different types of these capsomeres : 12 of the so-called pentons and 240 of the hexones . The hexons (8-10 nm) consist of a trimer of the hexon protein (virus protein II, molecular weight 120 kDa ) and are stabilized in members of the mastadenovirus genus by further hexon- associated proteins at their contact points (VP IX, X, XI). On the inside of the hexons there are two further proteins (VP VI and VIII) that simultaneously interact with proteins of the innermost nucleoprotein complex. The VP VI is in a ring-shaped arrangement only on the five hexons, which are each positioned around a penton.
The pentons are located at the corners of the icosahedral symmetry (at the axis points of the five-pointed axis of symmetry) and consist of a pentamer of the penton base protein (VP III, 80 kDa) and the penton base-associated protein (VP IIIa, 66 kDa). The fibers ( spikes ), which are composed of trimers of the glycosylated fiber protein (VP IV, 62 kDa), attach to the pentons - depending on the species, between 9 and 77.5 nm long . Within the genus Aviadenovirus , the fibers consist of two different fiber proteins.
The fiber protein mediates the binding to the surface of the host cell and induces the group-specific antibodies . Because of their fiber structure, adenoviruses can avoid a disadvantage of non-enveloped viruses compared to enveloped ones: Non- enveloped viruses can usually only adapt to a new host or a new target cell (thus a new receptor ) by mutating their outermost capsid proteins; however, these mutations can lead to the instability of the capsid or the complete loss of its packability. The adenoviruses, on the other hand, have the option of developing new variants simply by mutating the fiber structures and keeping the complex capsid unchanged.
The interior (core) of the adenoviruses is filled with a nucleoprotein complex, which consists of the double-stranded, linear DNA genome to which the basic proteins VP VII and X (in the mastadenovirus genus also VP V) are attached. In addition, there is a covalently bound protein, the so-called terminal protein (TP) , at both 5 'ends of the dsDNA .
The genome is between 26 and 45 kbp long and contains repetitive sequences at both ends , the so-called ITRs ( inverted terminal repeats ) . Within the family, the central part of the genome, which predominantly codes for the structural proteins of the capsid, is very conserved . However, the genera differ significantly in the DNA sequence and their gene products at the ends of the genome.
Systematics
The system according to ICTV (as of November 2018) includes u. a. the following species:
- Genus atadenovirus
- Genus aviadenovirus
- Genus ichtadenovirus
- Sturgeon ichtadenovirus A alias White sturgeon adenovirus in white sturgeon
- Genus mastadenovirus
- Human mastadenovirus A - F (HAdV-A to HAdV-F) in humans
- Bovine mastadenovirus A to C in cattle
- Canine mastadenovirus A
- Equine mastadenovirus A in horses
- Ovine mastadenovirus A and B in sheep
- Porcine mastadenovirus A in pigs
- Simian mastadenovirus A in monkeys
- Tree shrew mastadenovirus A in Tupaias
- “ Caprines adenovirus ” (Goat adenovirus 2, GAdV-2) in goats
- " Guinea Pig Adenovirus " in guinea pigs
- " Ovine adenovirus C " (Ovine adenovirus 6, OAdV-6) in sheep
- Genus Siadenovirus
- Frog siadenovirus A alias frog damageovirus 1 in frogs
- Great tit siadenovirus A with great tits
- Penguin siadenovirus A in penguins
- Raptor siadenovirus A in birds of prey
- Skua siadenovirus A at skuas
- Turkey siadenovirus A also known as turkey adenovirus A in turkeys
Chimpanzee adenoviruses : Adenoviruses isolated from chimpanzees are classified into “human” adenovirus species due to their great similarity to certain human adenoviruses (HAdVs). Thus, the simian adenoviruses are SADV-22 to-25 SADV to the species human Mastadenovirus E and SADV-21 to the species human Mastadenovirus B .
Similarities to other viruses
The phage PRD1 (family Tectiviridae ) shows striking similarities with adenoviruses in the structure of the capsid and the tail fibers. The arrangement of some genes on the genome of this phage (DNA polymerase, terminal protein) and the presence of two ITRs ( inverted terminal repeats ) also shows analogies, which indicates a phage- historical connection with the adenoviruses. Both families were therefore placed in the same class Tectiliviricetes by the ICTV in March 2020 .
In plants and fungi , a linear plasmid is found, partly in the cytoplasm or within the mitochondria (e.g. the “killer plasmid” of yeast), which shows a similar arrangement of the genes for the ITRs, the polymerase and the terminal protein .
The tail fibers of many members of the Adenoviridae and the Coxsackievirus B use the same receptor CAR ( C oxsackie- A denovirus- R ezeptor) for the detection of target cells. In addition, the adenoviral tail fibers have structural similarities to an attachment protein within the Reoviridae .
Applications
Human adenoviruses (especially type 5 from species C) are a gene therapy vector that is widely used in laboratories.
- In the future, adenoviral vectors are to be used in cancer therapy in particular. Genetically engineered vaccines are tested against infection with other viruses, as type 5 against Ebola virus and SARS-CoV-2 ( COVID-19 ).
- Non-replicating vectors derived from chimpanzee adenoviruses (ChAdOx1) are also tested against SARS-CoV-2.
- According to the authors, mutations of RPE65 that cause a disease of the retina ( Leber's congenital amaurosis ) were successfully treated in 2017 using adenovirus-based gene therapy (with vector AAV2-hRPE65v2).
- Since 2020, the Tübingen University Hospital has been testing how adenoviruses can be used for gene therapy in cases of complete color blindness ( achromatopsia ) (caused by a defective CNGA3 gene). The team named the vector they developed as AAV8.CNGA3 (adeno-associated virus with GNGA3 gene). Particularly with young patients, given the right conditions, there is a good chance of success.
Health consequences for humans
Human adenoviruses cause a variety of different diseases. The different species A – G of the adenoviruses cannot be clearly assigned to one clinical picture. The diseases that are triggered by human adenoviruses can range from mild to severe respiratory infections , through disseminated (distributed) infections in immunocompromised children, as well as diarrhea . Above all, the human adenovirus (type 19) is known to cause keratoconjunctivitis (inflammation of the eyes with involvement of the cornea). According to a US study, adenovirus 36 is said to induce human adipose tissue to transform into particularly large fat cells, which can cause obesity.
literature
- MB Matthews, T. Shenk: Adenovirus virus-associated RNA and translational control. In: Journal of Virology , 65, 1991, pp. 5657-5662.
- CM Fauquet, MA Mayo u. a .: Eighth Report of the International Committee on Taxonomy of Viruses. London / San Diego 2004.
- David M. Knipe, Peter M. Howley (Eds.): Fields' Virology . 4th edition. Philadelphia 2001.
Individual evidence
- ↑ a b c d e f ICTV: ICTV Taxonomy history: Human mastadenovirus C , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ^ WP Rowe, RJ Huebner et al. a .: Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. In: Proceedings of the Society for Experimental Biology and Medicine. Volume 84, Number 3, December 1953, ISSN 0037-9727 , pp. 570-573, PMID 13134217 .
- ↑ U. Krech. In: Textbook of Medical Microbiology. 6th edition. Gustav Fischer Verlag, Stuttgart 1988, p. 640.
- ↑ Hans von Kress (ed.): Müller - Seifert . Pocket book of medical-clinical diagnostics. 69th edition. Published by JF Bergmann, Munich 1966, p. 1062.
- ↑ SIB: Atadenovirus , on: ViralZone
- ↑ SIB: Aviadenovirus , on: ViralZone
- ↑ SIB: Ichtadenovirus , on: ViralZone
- ↑ NCBI: Canine mastadenovirus A (species)
- ^ A b D. Raoult: Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses , 2005: The Double Stranded DNA Viruses, ( Mastadenovirus :) TENTATIVE SPECIES IN THE GENUS
- ↑ SIB: Siadenovirus , on: ViralZone
- ↑ 9th report / dsdna-viruses-2011 / w / dsdna viruses / 93 / adenoviridae ICTV 9th Report (2011): Adenoviridae ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ^ Eugene V. Koonin, Valerian V. Dolja, Mart Krupovic: Origins and evolution of viruses of eukaryotes: The ultimate modularity . In: Virology , May 2015, pp. 479-480. 2-25, Epub March 12, 2015, PMC 5898234 (free full text), PMID 25771806
- ↑ H. Tazawa, S. Kagawa, T. Fujiwara: Advances in adenovirus-mediated p53 cancer gene therapy. In: Expert opinion on biological therapy. Volume 13, Number 11, November 2013, ISSN 1744-7682 , pp. 1569-1583, doi: 10.1517 / 14712598.2013.845662 , PMID 24107178 .
- ↑ IVI, INOVIO, and KNIH to partner with CEPI in a Phase I / II clinical trial of INOVIO's COVID-19 DNA vaccine in South Korea . International Vaccine Institute. April 16, 2020. Accessed April 23, 2020.
- ↑ Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers - Full Text View. In: clinicaltrials.gov. April 24, 2020, accessed April 29, 2020 .
- ↑ Jana Zeh: Safe virus as carrier - vaccine from China shows immune reactions , on n-tv.de from May 26, 2020
- ↑ Feng-Cai Zhu, Yu-Hua Li, Xu-Hua Guan, Li-Hua Hou, Wen-Juan Wang, Jing-Xin Li et al. : Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomized, first-in-human trial , in: The Lancet of May 22, 2020, doi: 10.1016 / S0140-6736 (20) 31208-3
- ↑ A Study of a Candidate COVID-19 Vaccine (COV001) - Full Text View. In: clinicaltrials.gov. March 27, 2020, accessed April 15, 2020 .
- ^ Covid 19 Vaccine Trial. University of Oxford, accessed May 20, 2020 .
- ↑ S. Russell, J. Bennett, J. A. Wellman et al. : Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomized, controlled, open-label, phase 3 trial. In: Lancet 390 (10097) of July 13, 2017, pp. 849-860, doi: 10.1016 / S0140-6736 (17) 31868-8 , PMID 28712537
- ↑ Nadja Podbregar: First gene therapy against complete color blindness: Gene repair proves to be safe and effective in first clinical study , on: scinexx.de of May 7, 2020.
- ↑ M. Dominik Fischer, Stylianos Michalakis, Barbara Wilhelm, D. Zobor, R. Muehlfriedel, S. Kohl, N. Weisschuh, GA Ochakovski, R. Klein, C. Schoen, V. Sothilingam, M. Garcia-Garrido, L Kuehlewein, N. Kahle, A. Werner A, D. Dauletbekov D, F. Paquet-Durand, S. Tsang, P. Martus, T. Peters, M. Seeliger, KU Bartz-Schmidt, M. Ueffing, E. Zrenner, M. Biel, B. Wissinger: Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial . In: JAMA Ophthalmology . April 30, 2020, doi : 10.1001 / jamaophthalmol.2020.1032 , PMID 32352493 , PMC 7193523 (free full text).
- ↑ JP Lynch, M. Fishbein, M. Echavarria: Adenovirus. In: Seminars in respiratory and critical care medicine. Volume 32, Number 4, August 2011, ISSN 1098-9048 , pp. 494-511, doi: 10.1055 / s-0031-1283287 , PMID 21858752 (review).
- ↑ Obesity: The cold virus makes children fat. In: Focus Online . September 20, 2010, accessed March 17, 2015 .
- ↑ HN Na, H. Kim, JH Nam: Prophylactic and therapeutic vaccines for obesity. In: Clinical and experimental vaccine research. Volume 3, number 1, January 2014, ISSN 2287-3651 , pp. 37-41, doi: 10.7774 / cevr.2014.3.1.37 , PMID 24427761 , PMC 3890448 (free full text) (review).



