Viral vector
As Viral vectors specifically altered to viral particles referred to in the genetic engineering can be used for genetic material to infiltrate into target cells. These can be cells from a living organism or cells from a cell culture .
The transport of DNA into a cell with the help of a virus is called transduction , and the cells that carry such DNA are called transduced cells . Viral vectors are used in basic research and in gene therapy . Also vaccines based on viral vectors are already in use. The viral vector technique was first used in the 1970s and has been continuously developed since then. In contrast to virus-like particles (VLP) or liposomes with viral membrane proteins ( virosomes ), viral vectors contain a nucleic acid to be transferred . This nucleic acid is then either temporarily read until it is broken down, or it is present as an episome in the cell nucleus next to the genome, or it is permanently integrated into the genome .
Naturally occurring viral vectors are e.g. B. the Polydnaviridae .
Properties of viral vectors
In order to introduce genetic material into target cells by means of transduction using a viral vector , the desired DNA sequence must first be cloned into the genome of the virus . In most cases, certain DNA regions of the viral genome are replaced, so that the viruses are no longer replication-competent , as they z. B. regulatory sequences or the genes for enzymes, capsid or membrane proteins are missing. Such vectors can no longer multiply themselves. Infectious particles can only be produced if complementation in the cell line that produces the vectors provides appropriate information, e.g. B. for the missing proteins and nucleic acids are provided separately. Replication-deficient vectors have the advantage that they cannot multiply in the target cell that does not contain this information and are therefore used in preference to uncontrolled replication-competent vectors. However, through homologous recombination in the packaging cell line, replication-competent viruses can arise again to a certain extent. To avoid this, the genetic material is often changed in the course of the vector design in such a way that they are replication-incompetent in as many respects as possible, e.g. B. by multiple deletions . If the genetic information is distributed over two different plasmids, it is referred to as a two-plasmid system . In order to further increase the safety of the vectors, the genetic information is also distributed over three or more different plasmids, which then each have to be transfected together to produce the vector. In addition to the vector design, a protein design can also be done. This allows the targeted adaptation of various properties of the transgene and its expressed protein .
Requirements for a viral vector are:
- Safety and low genotoxicity
- Low cytotoxicity and symptoms
- stability
- Cell type specificity
- Size of the genetic packaging capacity
While viral vectors for gene therapy of genetic defects a persistent gene expression to produce, are used for immunization against infectious diseases , for phage therapy of partially antibiotic-resistant bacteria , for the treatment of tumors with oncolytic viruses or for generating induced pluripotent stem cells transient viral vectors used.
Applications
Basic research
Viral vectors were originally developed as an alternative to the transfection of naked DNA for molecular genetic experiments. Compared to traditional methods such as calcium phosphate transfection, transduction can ensure that almost 100% of the cells receive the genetic material without significantly impairing the viability of the cells. In addition, some viruses and also the viral vectors derived from them are able to integrate their DNA into the genome of the target cell, so that the expression of the DNA can be stably anchored and can even be passed on to daughter cells. Since the production of viral vectors is very complex, the constantly improving transfection techniques are still the better solution for many applications.
Genes encoding proteins can be expressed with the help of viral vectors in order to examine the function of the respective protein. Vectors, especially retroviral vectors that express stable marker genes such as GFP , are used for permanent marking of cells in order to be able to track them and their offspring. This happens, for example, with xenotransplants .
Genes cloned into the vector, which code for shRNAs and miRNAs , can be used for RNA interference experiments.
Gene therapy
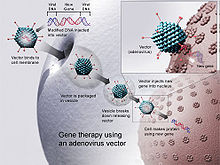
Various gene therapy studies have been carried out since the beginning of the 1990s, in which different viral vectors were used. With the help of these vectors, the defective gene, for example the dysfunctional inherited gene in the case of a monogenetic hereditary disease , is to be replaced by a healthy one. Different difficulties arise depending on which vector and which method is used. Great progress has already been made in vitro , but when used in patients these have always been accompanied by risks, which is why gene therapy has not yet found widespread use. The patient's immune reactions to the vector can cause complications, as in the case of Jesse Gelsinger, who was treated with an adenoviral vector in 1999. For these reasons it will be necessary to develop a suitable and specific viral vector for each disease that is to be treated by gene therapy.
Retroviral vectors, for example, integrate their genomes into the genome of the host cell. The individual integration takes place at a random location in the genome, but overall, depending on the virus type, certain locations such as active genes in HIV or promoter regions in MLV are strongly preferred. At these integration sites, the virus genome can interrupt genes of the host cell or activate genes that are naturally inactive, whereby it is assumed that this happens more or less frequently depending on the integration pattern of the respective virus. This can lead to degeneration of the cell and thus to tumor formation, as happened in a gene therapy study by Severe Combined Immunodeficiency (SCID), which was carried out in France in 1999. Four of the 10 children treated developed leukemia as a result of the treatment.
Vaccines
Transient viruses , which carry DNA from pathogens for transient expression, are currently being tested as vaccines against pathogens . The viral vector brings the DNA of the pathogen into the cell where it is expressed. The technique is thus an extension of the DNA vaccination .
history
In 1976, Paul Berg used a modified SV40 virus containing bacteriophage lambda DNA to infect monkey kidney cells that had been kept in cell culture. Retroviral vectors containing marker genes have been used since the mid-1980s to mark cells with them and to be able to follow their development within an organism. This made it possible to imagine using genes as therapeutic agents in target cells.
Types of viral vectors
Retroviruses
Retroviruses are currently at the center of gene therapy research and, due to their properties, are the most widely used persistent vectors. Recombinant retroviruses can integrate stably into the host genome and are currently the only instrument for the permanent cure of monogenetic hereditary diseases. Almost 300 clinical studies have been carried out so far (2007) with retroviral vectors.
Simple retroviruses such as B. Murine leukemia virus belonging to the genus of gamma retroviruses , depend on the dissolution of the cell nucleus during mitosis in order to integrate into the host genome and are therefore not suitable for the transduction of dormant cells , such as nerve cells. Because gamma retroviral vectors carry the risk by the strong U3 enhancer elements in their LTRs activating potential Onkongene near their integration site, were called SIN vectors (of s elf in activating developed), which these enhancers in the 3 'LTR -Area are missing. After reverse transcription and the integration of the provirus into the host genome, these are also missing in the 5 'LTR region. It has been shown that this reduces the risk of proto-oncogene activation, but it cannot be completely prevented even with SIN vectors. The tropism of a viral vector can be altered by pseudotyping , e.g. B. with the glycoprotein of the vesicular stomatitis virus ( VSV-G ).
Lentiviruses
Lentiviruses are a genus within the retroviruses. In contrast to gamma retroviruses, they can also replicate in resting cells and therefore have a potentially broader range of applications. The more complex biology of the lentiviruses is the reason why the development of vectors from lentiviruses is more difficult and why these vectors only provided usable results such as high titers after a longer lead time . The first clinical study published in November 2006, in which lentiviral vectors were used to suppress the replication of HIV-1 in patients with AIDS , was positive. SIN vectors have also been generated from lentiviral vectors, which represent an important advance in safety.
Foamy viruses
Foamy viruses are a zoonotic subfamily within the retroviruses. An advantage over simple retroviruses is, for example, the large foamy virus genome, so that larger therapeutic genes can also be packaged. Compared to lentiviruses, the general apathogenicity of foamy viruses should be emphasized, so that even with replication-competent vectors a low risk can be assumed. Foamy viruses also have other desirable properties, such as B. that, in contrast to orthoretroviruses, due to the genome -dependent assembly of the virion and the Env-dependent release, no virus-like particles that are unusable in gene therapy are formed and less active genes or transcription start sites are preferred for integration than in MLV or HIV. One problem, however, is the lack of pseudotyping of foamy viruses, for example. SIN vectors are also used with foamy viruses.
Adenoviruses
Unlike the lentiviruses, adenoviruses do not integrate into the cell's genome and are not replicated during cell division. Their use for basic research is therefore limited. Their main area of application is immunization or gene therapy when only temporary expression of the target gene is desired. This is the case with vaccines such as cAd3-ZEBOV and in combating tumors with oncolytic viruses . In the third generation adenoviral vectors, the production is virions of cofactors in a packaging cell line dependent. AdV vectors also induce vector immunity , so that one serotype can only be used once per patient.
Adeno-associated viruses
The adeno-associated virus (AAV) to induce human asymptomatic co-infections during adenoviral infection and be used to create persistent viral vectors. The adeno-associated viral vectors have a maximum DNA length of eight kilobases (single-stranded), which can still be packaged in virions . If self-complementary DNA is used, the maximum length of a transgene is reduced to five kilobases, and the virus titer decreases with lengths above this . Since, as with all viral vectors, antibodies against the viral proteins (in this case against capsid proteins ) arise in the context of an innate and an adaptive vector immunity, so that one serotype can only be used once per patient to avoid a premature breakdown of the vector or excessive immune reactions continuously developing new serotypes. However, integrations of AAV vectors have also been found in transcription-active areas of the genome , which can contribute to the development of tumors.
Vesicular stomatitis virus
The vesicular stomatitis virus is a virus which in humans produces little symptoms and to generate transient, but replication-competent is vectors used for vaccination purposes, for. B. VSV-EBOV .
Newcastle Disease Virus
The Newcastle Disease virus is used as a transient vector for vaccination purposes and as an oncolytic virus.
Modified Vaccinia Ankara
The modified vaccinia Ankara (MVA) is an attenuated smallpox virus , which as since the 1970s smallpox vaccine is approved and has been used mainly in Bavaria. After adding more antigens, it is used as a transient vector for vaccination against other diseases. Because of its vector immunity, the MVA is only effective in vaccinates who have not previously received a smallpox vaccination.
Alphavirus replicon
The replicon of the alphavirus is a transient vector that is replicated in the cytosol (replication-competent). Non-human pathogenic strains are used as alphaviruses. The RNA of the alphavirus replicon can also be transfected without a virus envelope or capsid . Alphavirus replicons produce a cytopathic effect . The period of action is lengthened by the replication, but slowly ended by the cytopathic effect.
Baculoviruses
Baculoviral vectors are used in insect cell cultures for the production of AAV vectors, among other things. The application in cell culture (without adaptive immune system ) enables continuous culture even with transient viruses. The use of insect cells reduces the risk of contamination with other human pathogens , which as a rule cannot infect insect cells. Insect cells also grow at room temperature and without CO 2 gassing, but sometimes show different glycosylation patterns of their proteins. There are also mammalian cell baculovirus vectors.
swell
- ^ E. Hoffmann, G. Neumann, Y. Kawaoka , G. Hobom, RG Webster : A DNA transfection system for generation of influenza A virus from eight plasmids. In: Proc Natl Acad Sci USA . Volume 97 (11), 2000, pp. 6108-6113. PMID 10801978 ; PMC 18566 (free full text).
- ↑ M. Stadtfeld u. a .: Induced Pluripotent Stem Cells Generated Without Viral Integration. science-online, September 25, 2008, doi: 10.1126 / science.1162494
- ^ JC Pagès, T. Bru: Toolbox for retrovectorologists. In: J Gene Med. Volume 6 Suppl 1, 2004, pp. S67-S82. PMID 14978752 .
- ↑ Reports of a second serious adverse event in a clinical trial of gene therapy for X-linked severe combined immune deficiency (X-SCID) ( Memento of October 28, 2008 in the Internet Archive ), press release of the European Society of Gene Therapy (ESGT) on the fourth leukemia case, January 18, 2003.
- ↑ SP Goff, P. Berg: Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells. In: Cell . 9, 1976, pp. 695-705. PMID 189942
- ^ R. Mann, RC Mulligan, D. Baltimore: Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. In: Cell. 33 (1), May 1983, pp. 153-159. PMID 6678608
- ↑ FD Bushman: Retroviral integration and human gene therapy. In: J Clin Invest. 117 (8), Aug 2007, pp. 2083-2086. PMID 17671645
- ↑ JD Sürth, V. Labenski, A. Schambach: Alpha Retro Viral Vectors: From a Cancer-Causing Agent to a Useful Tool for Human Gene Therapy. In: Viruses. Volume 6, Number 12, 2014, pp. 4811-4838, ISSN 1999-4915 . doi: 10.3390 / v6124811 . PMID 25490763 .
- ↑ ViralZone: Gammaretrovirus. Retrieved February 13, 2017 (American English).
- ↑ L. Biasco, C. Baricordi, A. Aiuti: Retroviral integrations in gene therapy trials. In: Mol Ther. Volume 20 (4), 2012, pp. 709-716. doi: 10.1038 / mt.2011.289 . PMID 22252453 ; PMC 3321603 (free full text).
- ^ KI Lim: Retroviral integration profiles: their determinants and implications for gene therapy. In: BMB Rep. Volume 45 (4), 2012, pp. 207–212. PMID 22531129 .
- ↑ U. Modlich, J. Bohne, M. Schmidt, C. von Kalle, S. Knöss, A. Schambach, C. Baum: Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. In: Blood . 108 (8), Oct 15, 2006, pp. 2545-2553. Epub 2006 Jul 6. PMID 16825499
- ↑ D. Zychlinski, A. Schambach, U. Modlich, T. Maetzig, J. Meyer, E. Grassman, A. Mishra, C. Baum: Physiological Promoters Reduce the Genotoxic Risk of Integrating Gene Vectors. In: Mol Ther. Feb 19, 2008. PMID 18388936
- ↑ J. Cronin, XY Zhang, J. Reiser: Altering the tropism of lentiviral vectors through pseudotyping. In: Current gene therapy. Volume 5, Number 4, August 2005, pp. 387-398, ISSN 1566-5232 . PMID 16101513 . PMC 1368960 (free full text).
- ^ T. Kafri: Gene delivery by lentivirus vectors. An overview. In: Methods Mol Biol. Volume 246, 2004, pp. 367-390. PMID 14970605 .
- ^ DB Kohn: Lentiviral vectors ready for prime-time. In: Nat Biotechnol . Volume 25 (1), 2007, pp. 65-66. PMID 17211402 .
- ↑ T. Iwakuma, Y. Cui, LJ Chang: Self-Inactivating Lentiviral Vectors with U3 and U5 Modifications. In: Virology. Volume 261 (1), 1999, pp. 120-132. PMID 10441560 .
- ^ Q. Yang, A. Lucas, S. Son, LJ Chang: Overlapping enhancer / promoter and transcriptional termination signals in the lentiviral long terminal repeat. In: Retrovirology. 4, 2007, p. 4, doi: 10.1186 / 1742-4690-4-4 .
- ↑ M. Linial: Why aren't foamy viruses pathogenic? In: Trends Microbiol. Volume 8, 2000, pp. 284-289.
- ↑ D. Lindemann, A. Rethwilm: Foamy virus biology and its application for vector development. In: Viruses. Volume 3 (5), 2011, pp. 561-585. PMID 21994746 ; PMC 3185757 (free full text).
- ↑ GD Trobridge, DG Miller, MA Jacobs, JM Allen, HP Kiem, R. Kaul, DW Russell: Foamy virus vector integration sites in normal human cells. In: Proc. Natl. Acad. Sci. USA Volume 103, 2006, pp. 1498-1503.
- ↑ A. Nowrouzi, M. Dittrich, C. Klanke, M. Heinkelein, M. Rammling, T. Dandekar, C. von Kalle, A. Rethwilm: Genome-wide mapping of foamy virus vector integrations into a human cell line. In: J Gen Virol. Volume 87, 2006, pp. 1339-1347.
- ↑ TR Bauer, JM Allen, M. Hai, LM Tuschong, IF Khan, EM Olson, RL Adler, TH Burkholder, YC Gu, D. Russell u. a .: Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. In: Nat. Med. Volume 14, 2008, pp. 93-97.
- ↑ T. Pietschmann, M. Heinkelein, M. Heldmann, H. Zentgraf, A. Rethwilm, D. Lindemann: Foamy virus capsids require the cognate envelope protein for particle export. In: J. Virol. Volume 73, 1999, pp. 2613-2621.
- ↑ T. Wiktorowicz, K. Peters, N. Armbruster, AF Steinert, A. Rethwilm: Generation of an improved foamy virus vector by dissection of cis-acting sequences. In: J. Gen. Virol. Volume 90, 2009, pp. 481-487.
- ↑ G. Trobridge, N. Josephson, G. Vassilopoulos, J. Mac, DW Russell: Improved foamy virus vectors with minimal viral sequences. In: Mol. Ther. Volume 6, 2002, pp. 321-328.
- ↑ JD Bassett, SL Swift, JL Bramson: Optimizing vaccine-induced CD8 (+) T-cell immunity: focus on recombinant adenovirus vectors. In: Expert Rev Vaccines. 10 (9), 2011, pp. 1307-1319. PMID 21919620 .
- ^ AU Ahmed, IV Ulasov, RW Mercer, MS Lesniak: Maintaining and loading neural stem cells for delivery of oncolytic adenovirus to brain tumors. In: Methods Mol Biol. Volume 797, 2012, pp. 97-109. PMID 21948472 .
- ^ E. Dormond, AA Kamen: Manufacturing of adenovirus vectors: production and purification of helper dependent adenovirus. In: Methods Mol Biol. Volume 737, 2011, pp. 139-156. PMID 21590396 .
- ^ AC Silva, C. Peixoto, T. Lucas, C. Küppers, PE Cruz, PM Alves, S. Kochanek: Adenovirus vector production and purification. In: Curr Gene Ther. Volume 10 (6), 2010, pp. 437-455. PMID 21054247 .
- ↑ B. Thaci, IV Ulasov, DA Wainwright, MS Lesniak: The challenge for gene therapy: innate immune response to adenoviruses. In: Oncotarget . Volume 2 (3), 2011, pp. 113-121. PMID 21399236 ; PMC 3092742 (free full text).
- ↑ YS Ahi, DS Bangari, SK Mittal: Adenoviral vector immunity: its implications and circumvention strategies. In: Curr Gene Ther. Volume 11 (4), 2011, pp. 307-320. PMID 21453277 .
- ↑ G. Murlidharan, RJ Samulski, A. Asokan: Biology of adeno-associated viral vectors in the central nervous system. In: Frontiers in molecular neuroscience. Volume 7, 2014, p. 76, ISSN 1662-5099 . doi: 10.3389 / fnmol.2014.00076 . PMID 25285067 . PMC 4168676 (free full text).
- ^ DM McCarty: Self-complementary AAV vectors; advances and applications. In: Mol Ther. Volume 16 (10), 2008, pp. 1648-1656. PMID 18682697 .
- ↑ a b R. M. Kotin: Large-scale recombinant adeno-associated virus production. In: Hum Mol Genet . Volume 20 (R1), 2011, pp. R2-R6. PMID 21531790 ; PMC 3095058 (free full text).
- ↑ F. Mingozzi, KA High: Immune responses to AAV in clinical trials. In: Curr Gene Ther. Volume 11 (4), 2011, pp. 321-330. PMID 21557723 .
- ^ I. Kwon, DV Schaffer: Designer gene delivery vectors: Molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. In: Pharmaceut Res. Volume 25, 2008, pp. 489-499.
- ^ E. Ayuso, F. Mingozzi, F. Bosch: Production, purification and characterization of adeno-associated vectors. In: Curr Gene Ther. Volume 10 (6), 2010, pp. 423-36. PMID 21054248 .
- ^ J. Wang, SM Faust, JE Rabinowitz: The next step in gene delivery: molecular engineering of adeno-associated virus serotypes. In: Journal of Molecular and Cellular Cardiology . Volume 50 (5), 2011, pp. 793-802. PMID 21029739 .
- ^ DR Deyle, DW Russell: Adeno-associated virus vector integration. In: Curr Opin Mol Ther. Volume 11 (4), 2009, pp. 442-447. PMID 19649989 ; PMC 2929125 (free full text).
- ↑ A. Donsante, DG Miller, Y. Li, C. Vogler, EM Brunt, DW Russell, MS Sands: AAV vector integration sites in mouse hepatocellular carcinoma. In: Science. Volume 317 (5837), 2007, p. 477. PMID 17656716 .
- ↑ JS Richardson, JD Dekker, MA Croyle, GP Kobinger: Recent advances in Ebolavirus vaccine development. In: Human vaccines. Volume 6, Number 6, June 2010, pp. 439-449, ISSN 1554-8619 . PMID 20671437 .
- ↑ V. Schirrmacher, P. Fournier: Multimodal cancer therapy involving oncolytic Newcastle disease virus, autologous immune cells, and bi-specific antibodies. In: Frontiers in oncology. Volume 4, 2014, p. 224, ISSN 2234-943X . doi: 10.3389 / fonc.2014.00224 . PMID 25309868 . PMC 4160967 (free full text).
- ^ PF McKay, AV Cope, JF Mann, S. Joseph, M. Esteban, R. Tatoud, D. Carter, SG Reed, J. Weber, RJ Shattock: Glucopyranosyl lipid A adjuvant significantly enhances HIV specific T and B cell responses elicited by a DNA-MVA-protein vaccine regimen. In: PloS one. Volume 9, number 1, 2014, p. E84707, ISSN 1932-6203 . doi: 10.1371 / journal.pone.0084707 . PMID 24465426 . PMC 3900398 (free full text).
- ↑ T. Osada, MA Morse, A. Hobeika, HK Lyerly: Novel recombinant alphaviral and adenoviral vectors for cancer immunotherapy. In: Seminars in oncology. Volume 39, Number 3, June 2012, pp. 305-310, ISSN 1532-8708 . doi: 10.1053 / j.seminoncol.2012.02.013 . PMID 22595053 . PMC 3607360 (free full text).
- ↑ MM Segura, AA Kamen, A. Garnier: Overview of current scalable methods for purification of viral vectors. In: Methods Mol Biol. Volume 737, 2011, pp. 89-116. PMID 21590394 .
- ↑ TA Kost, JP Condreay: Recombinant baculoviruses as mammalian cell gene-delivery vectors. In: Trends in biotechnology. Volume 20, Number 4, April 2002, pp. 173-180, PMID 11906750 .
literature
- Gary L. Buchschacher, Jr .: Lentiviral Vector Systems for Gene Transfer Kluwer Academic / Plenum Publishers, New York 2003, ISBN 0-306-47702-5 .
- Curtis A. Machida (Ed.): Viral Vectors for Gene Therapy. Methods and Protocols Humana Press, 2002, ISBN 1-58829-019-0 .
Web links
- Virus Vectors & Gene Therapy: Problems, Promises & Prospects. ( Memento from October 26, 2014 in the web archive archive.today )