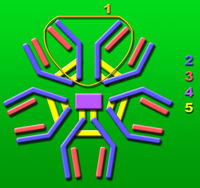
Immunoglobulin M
The immunoglobulin M ( IgM ) is an antibody molecule , consisting of five Y-shaped sub-units which are joined together at the long ends. The connection of the individual antigen-binding molecules takes place through additional intermolecular disulfide bridges and the so-called J-chain. Its concentration in the blood serum is 1.5 mg / ml.
The basic unit consists of two long ("heavy", also H-chain (from English heavy)) and two short ("light", also L-chain) protein chains and has the shape of a "Y". At the short ends of the Y are the binding sites that can bind to antigens (foreign bodies, for example specific surface structures of bacterial cells ).
IgM has a function as the earliest immunoglobulin produced in the course of an immune response to activate the complement system , which is why an increased IgM antibody level in a blood test indicates a current immune response - i.e. a current infection .
Natural IgM
Natural IgM antibodies are secreted by B1 cells . In humans, these are CD20 +, CD27 +, CD43 +, and CD70 +. In addition to their role in defense against invading microbes, they are also involved in tissue homeostasis through the clearance of apoptotic and altered cells through complement-dependent mechanisms. They inhibit inflammation, remove malformed cells and regulate both the pathogenic, autoreactive antibodies of the IgG isotype and the autoantibody-producing B cells. In the future, this may open up new therapeutic possibilities such as stimulating the production of natural IgMs or the pharmacological substitution of mono- or polyclonal IgM preparations. IgM antibodies appear as the first isotype in the ontogenesis of B cells and form the first immunoglobulin that is secreted during the antigen-specific immune response.
literature
- Charles Janeway , Paul Travers, Mark Walport, Mark Shlomchik: Immunology. 5th edition, Spektrum Akademischer Verlag, Heidelberg 2002, ISBN 3-8274-1079-7 ; Online version in English , 5th edition, 2001.
- V. Kaveri, Gregg J. Silverman, Jagadeesh Bayry: Natural IgM in Immune Equilibrium and Harnessing Their Therapeutic Potential. J Immunol 2012; 188: 939-945.