Daunorubicin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
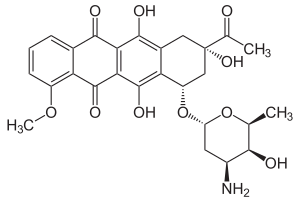
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Daunorubicin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 27 H 29 NO 10 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 527.52 g mol −1 | |||||||||||||||||||||
| Melting point |
208-209 ° C |
|||||||||||||||||||||
| solubility |
slightly soluble in water (39.2 mg l −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Daunorubicin (DNR) is a natural glycoside and antibiotic from the group of anthracyclines . It is used as a cytostatic agent as part of the combination chemotherapy for acute leukemia.
Origin and manufacture
Daunorubicin is produced by Streptomyces peucetius and Streptomyces coeruleorubidus .
Mechanism of action
Daunorubicin is a DNA - intercalator . The planar structure causes the intercalation between the nucleobases of the DNA. This blocks the transcription of the DNA for the synthesis of RNA or the replication of the DNA during cell division. This effect is also mediated by the inhibition of topoisomerase II .
Daunorubicin also works via redox reactions . Daunorubicin is activated to an intermediate product with properties of a free radical. This daunorubicin radical transfers its electrons to molecular oxygen , which subsequently leads to the formation of cytotoxic superoxide and hydroxyl radicals . Apart from their toxic effects, these can also induce DNA strand breaks. Because of the abundance of oxygen and catalytically active iron , the heart muscle is particularly affected by this mechanism of action.
Pharmacokinetics
Interactions
When heparin and daunorubicin are administered together , the two substances precipitate in the infusion solution. A separate feed is therefore required.
The action of daunorubicin hinders the absorption of ciprofloxacin from the intestines. This results in a loss of effectiveness of ciprofloxacin when administered orally.
application areas
Daunorubicin is used as a cytostatic drug to treat cancer . It is not used as an immunosuppressant in autoimmune diseases .
Adults
Daunorubicin is used in the treatment of acute myeloid leukemia and acute lymphoblastic leukemia . The treatment takes place almost exclusively in combination with other cytostatics.
Children and young people
Daunorubicin is used in the treatment of acute myeloid leukemia and acute lymphoblastic leukemia (ALL). The treatment takes place almost exclusively in combination with other cytostatics.
In ALL, daunorubicin is used at the beginning of treatment (so-called induction phase).
Daunorubicin is given as an intravenous infusion only .
Side effects
The side effects of daunorubicin are explained by the growth-inhibiting and cell-toxic effects or the mechanism of action of daunorubicin. In principle, all tissues that have a high rate of growth or cell division (mucous membranes, hair, blood formation in bone marrow ) are preferentially damaged. A special feature is the cardiotoxicity (damage to the heart) of daunorubicin, which is partly explained by the increased presence of oxygen ( myoglobin , blood circulation) and iron (catalytic effect, Fenton reaction ) in the heart muscle .
Gastrointestinal tract
Damage to the mucous membrane ( mucositis ). The rapidly growing mucous membranes, especially those of the gastrointestinal tract, are damaged by the action of daunorubicin. The extent of the damage can be minor; The damage to the mucous membranes is usually so pronounced that ulcers develop . Damage to the mucous membrane causes pain in the mouth (stomatitis) or stomach on the one hand due to the inadequate barrier function of the mucous membrane (which may require morphine), and on the other hand there is a risk of infection due to the passage of pathogenic bacteria, viruses and fungi from the mouth or intestinal contents into the Blood stream increased significantly.
Nausea and vomiting . Some of these side effects are triggered directly by daunorubicin; some of them can also be the result of damage to the mucous membrane. Nausea and vomiting as a result of or during daunorubicin therapy require antiemetic therapy with, for example, ondansetron , tropisetron or granisetron . The extent of vomiting can be so severe that it leads to disorders of the water-salt balance that require treatment.
Abdominal pain . As a result of the damage to the mucous membrane of the intestine, after daunorubicin is administered, abdominal pain usually occurs at the same time as diarrhea.
Diarrhea . Diarrhea occurs as a result of daunorubicin administration when the damage to the mucous membrane, especially in the intestine, is so severe that the intestinal function is significantly impaired. The diarrhea can be so severe that it can lead to changes in the water and salt balance that require treatment. With simultaneous thrombopenia, diarrhea can lead to bleeding that requires treatment.
Blood formation, bleeding and infection
Leukopenia , anemia , thrombopenia . Daunorubicin damages blood formation in the bone marrow when given. This results in a decrease in the number of leukocytes (leukopenia) and thrombocytes (thrombopenia), less of the erythrocytes (anemia). With leukopenia, neutropenia also occurs. The nadir (low point) is reached 8-10 days after daunorubicin administration. 14–21 days after daunorubicin administration, the bone marrow and the blood formation recovered.
Infections . As a consequence of leukopenia (especially neutropenia), infections occur more frequently during daunorubicin therapy or after administration of daunorubicin. These are mainly bacterial or mycotic. The infections can be serious or even life-threatening. In addition,infections with viruses can occuras a result of lymphopenia . These can also be serious or even life-threatening.
Bleeding . Thrombopenia after daunorubicin administration increases the risk of bleeding. The risk of bleeding can increase further if liver disorders(including daunorubicin) cause a decrease in the number of platelets and a decrease in the coagulation factors . The risk of bleeding is also significantly increased if there is extensive damage to the mucous membranes at the same time as thrombopenia.
Hair loss
Hair loss (alopecia). Analogous to the damage to the rapidly growing mucous membrane, hair growth is also disturbed by daunorubicin. The damage can vary between a halt in hair growth and complete hair loss. This side effect is mostly fully reversible after the end of therapy with daunorubicin or other cytostatics.
Cardiotoxicity
The heart-damaging ( cardiotoxic ) effect of daunorubicin essentially corresponds to that of other anthracyclines ( doxorubicin , epirubicin , idarubicin ) and anthracenediones ( mitoxantrone ). A distinction is made between an immediate type and a late type:
Acute cardiotoxicity . The so-called immediate type is characterized by arrhythmias , endomyocardial fibrosis , angina pectoris or peri- and / or myocarditis . It is independent of the dose and usually occurs during or immediately after administration of daunorubicin. Depending on the severity and damage, this side effect can be reversible or irreversible.
Cardiomyopathy (heart muscle damage). Cardiomyopathy usually occurs after repeated doses of daunorubicin. Its occurrence depends on the total dose of daunorubicin (and other anthracyclines or antracenedions ) administered. Weeks and months, usually even years, can elapsebetween the appearance of symptoms such as poor performance , pulmonary edema and heart failure and the administration of daunorubicin (late-type cardiotoxicity). In the meantime, there can be complete freedom from symptoms. The cumulative dose daunorubicin, increased cardiomyopathies be observed from the, is 450 to 550 mg / m 2 KOF (also up to 600 mg / m 2 BSA) in adults, 300-400 mg / m 2 KOF (also up to 450 mg / m 2 KOF) in children over 2 years of age or 10 mg / kg body weight in children under 2 years of age.
The course of a daunorubicin-related cardiomyopathy can be so severe that only a heart transplant can cure or improve the condition. Performing echocardiographies before each dose of daunorubicin is an effective measure for the early detection of an increased risk of cardiomyopathy: the ejection fraction (EF) is used as the parameter , with 28% denoting the lower limit of the normal range. If other cardiotoxic (harmful effects on the heart) drugs have been administered or if the chest has been irradiated beforehand, the cardiomyopathy can become more pronounced.
Contraindications (contraindications)
- pregnancy
- Lactation
- Vaccinations with live vaccines
- severe heart disease
Trade names
Cerubidine (CH), Daunoblastin (D, A), DaunoXome (A)
Web links
- BC Cancer Agency's monograph Daunorubicin as of June 1, 2004, freely accessible.
Individual evidence
- ↑ a b c Entry on daunorubicin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Datasheet Daunorubicin hydrochloride from Sigma-Aldrich , accessed on March 24, 2011 ( PDF ).