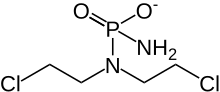
Cyclophosphamide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| 1: 1 mixture of ( R ) -form (left) and ( S ) -form (right) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Cyclophosphamide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 279.1 g mol −1 (cyclophosphamide monohydrate) |
|||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
41–45 ° C and 47–49 ° C (cyclophosphamide monohydrate) |
|||||||||||||||||||||
| solubility |
easily soluble in water (40 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
The oxazaphosphinane cyclophosphamide (trade name: Endoxan , manufacturer: Baxter ) is a medicinal substance and belongs to the group of nitrogen-mustard gas compounds with an alkylating effect . It is a cytostatic agent . In addition to cancer therapy, it is also used to treat particularly severe courses of autoimmune diseases such as systemic lupus erythematosus (SLE) , scleroderma , dermatomyositis ( MDA5 pos.), Vasculitides such as granulomatosis with polyangiitis , rheumatoid arthritis and experimentally in multiple sclerosis . Since cyclophosphamide only has a cytotoxic effect after activation in the liver, it is a prodrug .
history
In their search for a cancer drug, the chemists at Asta Medica synthesized derivatives of phosphamide mustard . The later cyclophosphamide was found in 1956 and patented in 1962.
Manufacturing
Cyclophosphamide is made from bis (2-chloroethyl) amine and phosphorus (V) oxychloride . The resulting phosphoric acid amide dichloride yields racemic cyclophosphamide on reaction with 3-amino-1-propanol in the presence of triethylamine .
pharmacology
Cyclophosphamide per se is not a cytostatic substance. When absorbed into the body and passed through the bloodstream through the liver, it is activated in it. The bioavailability is> 75% after oral administration, the elimination half-life is 3–12 hours. Enzymes of the cytochrome P450 system (especially CYP2B6) convert cyclophosphamide through hydroxylation to 4-hydroxycyclophosphamide (4-OH-cyclophosphamide). 4-OH-cyclophosphamide is in equilibrium with the tautomer aldophosphamide.
Aldophosphamide spontaneously splits off acrolein and becomes phosphoramide mustard (chloroethylphosphoric acid amide). The phosphoramide mustard is an active bifunctional alkylane and a zwitterion at a physiological pH (7.4): The phosphoramide mustard form only reaches cells with difficulty via the cell membrane , while the 4-OH-cyclophosphamide-aldophosphamide form easily enters the cells through the cell membrane. 4-OH-cyclophosphamide-aldophosphamide thus serves as a means of transport of phosphoramide mustard into the cells; the latter is significantly more cytotoxic than 4-OH-cyclophosphamide-aldophosphamide. Analogous to the substance mechlorethamine , phosphoramide mustard causes crosslinking connections between the individual DNA strands ( DNA crosslinking ) in cells .
Aldehyde dehydrogenases break down 4-OH-cyclophosphamide by oxidizing it to carboxyphosphamide. Cells that are rich in aldehyde dehydrogenases, such as early hematopoietic stem cells and megakaryocytes as well as stem cells in the mucous membranes, are less sensitive to the toxic effects of 4-OH-cyclophosphamide and phosphoramide mustard than cells without an abundant supply of aldehyde dehydrogenases. This difference in metabolic ability explains the relatively short duration of bone marrow toxicity with anemia , thrombopenia and leukopenia . It also explains the lower toxicity of the mucous membranes ( mucositis ) compared to other alkylating substances .
effectiveness
Cancers
Cyclophosphamide is used as a cytostatic agent in a number of malignant diseases (cancers) . It is mostly used in combination with other cytostatics.
Adults
- Breast cancer (CMF scheme: cyclophosphamide, methotrexate , 5-fluorouracil )
- Soft tissue sarcomas
- Hodgkin lymphoma
- Non-Hodgkin lymphoma
- Ewing's sarcoma
- Conditioning treatment before stem cell transplantation and immunotherapy
- Mobilization of stem cells for stem cell apheresis
Children and young people
- Medulloblastoma
- Soft tissue sarcomas such as rhabdomyosarcoma , leiomyosarcoma
- Acute lymphoblastic leukemia - in combination with cytarabine or methotrexate and asparaginase
- Hodgkin lymphoma
- Non-Hodgkin lymphoma
- Neuroblastoma
- Retinoblastoma
- Ewing's sarcoma
- Conditioning treatment before stem cell transplant
- Mobilization of stem cells for stem cell apheresis
- Severe and very severe aplastic anemia
multiple sclerosis
In several studies, a reduction in progression could be observed with good tolerability. Booster infusions were more effective than a single induction therapy.
There is currently no approval for the treatment of multiple sclerosis with cyclophosphamide .
Mechanism of action
Cyclophosphamide leads to single and double strand breaks in rapidly dividing cells. As a result, there are more CD8 suppressor cells and fewer CD4 helper cells in the blood of treated patients .
Side effects
A reduced number of leukocytes (dose-limiting bone marrow depression ), nausea and hair loss often occur . The risk of cancer, especially leukemia and bladder tumors, is increased especially with high cumulative doses. In addition, chemotherapy with cyclophosphamide can lead to hemorrhagic cystitis . In contrast, a protective substance is administered parallel to the cyclophosphamide administration: Mesna (mercapto-ethanesulfonate sodium). However, the usefulness of administering Mesna is quite controversial, especially in the case of cyclophosphamide therapies in the non-oncological area (for example in the therapy of granulomatosis with polyangiitis ), since the dosages used are significantly lower.
Furthermore, infertility can occur in women and men after administration of cyclophosphamide , which is why cryopreservation of sperm or egg cells should be considered before the first administration. Vaccinations with dead vaccines are not effective during cyclophosphamide treatment because of its immunosuppressive and cytostatic effects.
Contraindications (contraindications)
- Pregnancy . Pregnancy should be excluded before cyclophosphamide therapy; an existing pregnancy is a contraindication ( contraindication ) for the use of cyclophosphamide. Adequate contraception should be used during therapy to prevent pregnancy.
- Vaccinations with live vaccines . Because of their cytostatic and immunosuppressive effects, vaccinations with live vaccines during treatment with cyclophosphamide are potentially dangerous and should therefore be avoided.
Significance for psychoneuroimmunology
The ability of the immune system to be conditioned was first demonstrated by Robert Ader (1932–2011) using cyclophosphamide as an unconditioned stimulus. He was able to demonstrate a classically conditioned immunosuppressive effect of the substance in rat experiments. First a saccharin solution (neutral stimulus) paired with cyclophosphamide was presented. After the administration of sweetened water, the animals were injected with the nauseating immunosuppressive agent. As a result, in addition to a conditioned taste aversion to the saccharin solution, reduced antibody production and increased mortality were found in the conditioned test animals. I.e. the sweetener, which originally had no effect on immune function, triggered immunosuppressive effects comparable to those of cyclophosphamide after learning about conditioning. With these findings Ader founded the so-called psychoneuroimmunology , which deals with the influences of psychological and neural mechanisms on the immune system (especially on the immune defense).
Web links
- Monograph Cyclophosphamide British Columbia Cancer Agency ( October 30, 2007 memento in the Internet Archive ) Open to the public. Status 08/2006.
- US-American technical information cyclophosphamide ( Memento from April 11, 2008 in the Internet Archive ) as of 07/2000. Freely accessible.
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 460, ISBN 978-0-911910-00-1 .
- ↑ a b c Entry on cyclophosphamide in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Mechtild Wolf (ed.): Always an idea better: Researcher and inventor at Degussa ; Frankfurt am Main, Degussa AG 1998 (p. 172 f.)
- ↑ Entry on cyclophosphamide. In: Römpp Online . Georg Thieme Verlag, accessed on January 23, 2015.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dietmar Reichert: Pharmaceutical Substances , 4th edition (2000), 2 volumes published by Thieme-Verlag Stuttgart, p. 563, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ↑ TK Chang, GF Weber u. a .: Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. In: Cancer Research . Volume 53, Number 23, December 1993, pp. 5629-5637, PMID 8242617 .
- ↑ Klaus Aktories, Ulrich Förstermann, Franz Bernhard Hofmann, Klaus Starke (eds.): General and special pharmacology and toxicology . Founded by W. Forth, D. Henschler, W. Rummel. 10th edition. Urban & Fischer in Elsevier, Munich 2009, ISBN 978-3-437-42522-6 .
- ^ S1 guideline medulloblastoma in children and adolescents of the Society for Pediatric Oncology and Hematology (GPOH). In: AWMF online (as of 11/2012)
- ↑ C. Krishnan, AI Kaplin et al. a .: Reduction of disease activity and disability with high-dose cyclophosphamide in patients with aggressive multiple sclerosis. In: Archives of neurology. Volume 65, Number 8, August 2008, pp. 1044-1051, doi: 10.1001 / archneurol.65.8.noc80042 , PMID 18541787 , PMC 2574697 (free full text).
- ^ DM Harrison, DE Gladstone et al. a .: Treatment of relapsing-remitting multiple sclerosis with high-dose cyclophosphamide induction followed by glatiramer acetate maintenance. In: Multiple sclerosis. Volume 18, number 2, February 2012, pp. 202-209, doi: 10.1177 / 1352458511419701 , PMID 21865410 , PMC 3612927 (free full text).
- ^ PA Monach, LM Arnold, PA Merkel: Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review. In: Arthritis and rheumatism. Volume 62, number 1, January 2010, pp. 9-21, doi: 10.1002 / art.25061 , PMID 20039416 (review).
- ^ R. Ader, N. Cohen: Behaviorally conditioned immunosuppression. In: Psychosomatic medicine. Volume 37, Number 4, 1975, pp. 333-340, PMID 1162023 .