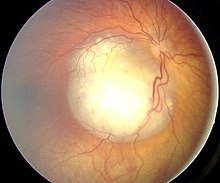
Retinoblastoma
| Classification according to ICD-10 | |
|---|---|
| C69.2 | Malignant neoplasm: retina |
| ICD-10 online (WHO version 2019) | |

The retinoblastoma is a malignant tumor in the retina of the eye, for the development of which mutations in both alleles of the retinoblastoma gene (located on chromosome 13 , band q14) are the basic prerequisite.
This tumor originates from genetically modified immature retinal cells and, if left untreated, leads to death. If the disease is recognized and treated early, the chances of recovery are good (approx. 95% of patients are cured). Since the retinoblastoma can only grow from immature retinal cells, this tumor very rarely occurs after the age of 5. For every 20,000 live births there is about one case of illness, which corresponds to about 60 cases per year in Germany. In the USA, around 350 cases are expected each year, and worldwide there are 5,000–8,000 new cases per year. A distinction must be made between hereditary and non-hereditary forms. The tumor occurs with the same frequency in girls and boys. Apart from humans, no animal species is known so far in which retinoblastoma occurs naturally.
root cause
About 45% of patients have the hereditary form of retinoblastoma. These patients are heterozygous for a mutation in the retinoblastoma gene (first mutation). These mutations are usually the result of new mutations in the germ line (sperm cell or egg cell) of a parent. There is an autosomal dominant inheritance with incomplete but high penetrance . This autosomal dominant inheritance is remarkable in that it differs significantly from other autosomal dominant inheritance. For retinoblastoma to develop, both alleles for the retinoblastoma gene (RB1) must be mutated; this is usually a property of autosomal recessive inherited diseases. In the case of hereditary retinoblastoma, however, in almost 95% of cases there is an additional second somatic mutation in retinal precursor cells when there is a first mutation in all body cells. The mutation-related loss of the second, normal allele in a precursor cell of the retina triggers the development of the tumor. As a result, the inheritance appears typically autosomal dominant with incomplete but high penetrance. Investigations on this were carried out by Alfred G. Knudson and published in 1971, after him this theory of tumor development is called " Knudson's two mutations theory ". It is now assumed that a third factor must be added in order for the tumor to develop. In this context, for example, changes in the chromosome structure are discussed, such as gains on the long arm of chromosome 1 or on the short arm of chromosome 6 .
Most patients with hereditary retinoblastoma have tumors in both eyes (bilateral retinoblastoma). Heterozygous carriers of a mutation in the retinoblastoma gene pass it on to their offspring with a risk of 50%. Children who have inherited such a mutation are at very high risk of developing retinoblastoma.
About 55% of patients have the non-hereditary form of retinoblastoma. In these patients, both mutations required for tumor development occur in body cells (somatic mutations), and both mutations must be present in one and the same cell. Since it is statistically quite unlikely that this double mutation in a child occurs independently in cells of the retina on both sides, sporadic retinoblastoma is rarely bilateral. Almost all patients with non-hereditary retinoblastoma have only one affected eye (unilateral retinoblastoma).
Since retinoblastoma usually occurs in young children under the age of five, retinoblastoma is also known as a child's eye tumor . The disease is mostly diagnosed earlier in children with bilateral retinoblastoma than in children with unilateral retinoblastoma; the average age at diagnosis is 23 months for the unilateral form and 12 months for the bilateral form. About 10% of retinoblastomas are diagnosed shortly after birth, about 50% within the first year of life, and about 90% by the age of 3.
Special forms
Trilateral retinoblastoma
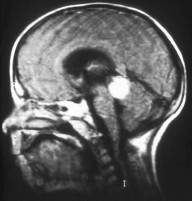
Trilateral retinoblastoma is a very rare hereditary retinoblastoma that occurs together with a brain tumor. This is an independent tumor, not a metastasis. The histology is similar to that of retinoblastoma; the prognosis is relatively unfavorable for the patient.
Retinoma (retinocytoma)
Retinoma is a benign retinal tumor that occurs in approximately 2% of patients with a mutation in the RB gene. It is probably a forerunner of retinoblastoma or the remains of a healed retinoblastoma after spontaneous regression. The histology is similar to that of retinoblastoma.
Symptoms
| symptom | frequency |
|---|---|
| Leukocoria | 56% |
| Strabismus | 20% |
| painful redness / glaucoma | 7% |
| Loss of vision | 5% |
| orbital cellulitis | 3% |
| unilateral mydriasis | 2% |
| Heterochromia iridis | 1 % |
| Hyphema | 1 % |
| white iris spots | 0.5% |
| Anorexia / developmental disorder | 0.5% |
It is not uncommon for the tumor to be noticed by so-called leukocoria . The (flash) light falling into the eye is not reflected by the retina with the underlying choroid, as is the case with the healthy eye (this results in the typical "red eye" on photographs), but by the retinoblastoma tissue (this results in a whitish-yellowish color Reflection of the light, see illustration). In retinoblastoma patients who later become clinically conspicuous due to other symptoms, the phenomenon of leukocoria can often already be observed on earlier photographs. If there is also tumor-related "blindness" ( amaurosis ) on the eye , it is also referred to as the "amaurotic cat's eye".
In addition to leukocoria, a squint ( strabismus ) of the eyes is the most common symptom. Painful reddening of the eye, glaucoma ( glaucoma ), partial loss of vision, inflammation in the eye socket ( orbital cellulitis ), very rarely a one-sided widening of the pupil (unilateral mydriasis ), discoloration of the iris ( heterochromia ), white is less common Iris spots or blood in the anterior chamber of the eye ( hyphema ).
Diagnosis
Common examination methods are, in particular, ophthalmoscopy and sonography . In certain cases, computed tomography or magnetic resonance imaging is also performed, and in the advanced stages of the disease, an examination of the liquor and bone marrow is also carried out in order to detect metastasis . The retinal dysplasia is to be distinguished .
Reese-Ellsworth Classification
This classification system , most widely used for retinoblastoma , was developed at Columbia Presbyterian Medical Center in the 1960s . This is not a classic staging system in which patients progress from Group I to the higher groups. Rather, it was designed to predict the prognosis of tumors after percutaneous radiation therapy : the higher the classification, the further forward a tumor sits in the eye and the worse the treatment success. With the increasing trend towards other therapies, this classification system is increasingly being called into question; however, other systems have not yet caught on.
- Yes - Solid tumor, <4 disc diameter, at or behind the equator
- Ib. - Multiple tumors, <4 disc Ø, at or behind the equator
- IIa. - Solid tumor, 4-10 disc Ø, at or behind the equator
- IIb. - Multiple tumors, 4-10 disc Ø, at or behind the equator
- IIIa. - All lesions in front of the equator
- IIIb. - Solid tumor> 10 disc diameter behind the equator
- IVa. - Multiple tumors,> 10 disc Ø
- IVb. - All rostral lesions of the ora serrata
- Va. - Tumor takes up over half of the retina
- Vb - Vitreous sowing
therapy
The chance of a cure and preservation of vision depends very much on the spread of the tumor in and outside the eye. The main goal in any case is to save the patient, which results in a complete removal of the tumor. Whenever possible, one tries to preserve the eyeball and as much of the eyesight as possible.
Enucleation
In the advanced stages of the disease, the only way to completely eliminate the tumor is to remove the affected eye. During this enucleation , the eye is surgically removed with as large a part of the optic nerve as possible (through which metastases can spread to the brain). The eye muscles and the rest of the contents of the orbit are preserved. After the enucleation, the tumor and optic nerve are examined histologically in order to be able to determine any metastasis.
After the eyeball has been removed, an implant is placed in the eye socket on which an eye prosthesis can be placed.
Percutaneous radiation therapy
The retinoblastoma is extremely sensitive to radiation . Therefore, percutaneous radiation therapy (irradiation of the tumor through the skin without the lens) was the standard therapy for a long time. However, it is now known that the risk of secondary tumors increases by a factor of 3-6 due to radiation (especially with hereditary retinoblastoma and radiation in the first year of life). Today, percutaneous radiation therapy is mainly used for vitreous seeding , metastasis or when chemotherapy has proven to be ineffective or incompatible. Possible side effects are radiation-related damage to the components of the eye such as the retina ( radiation retinopathy ), the optic nerve ( radiation optic neuropathy and optic atrophy), the lens ( radiation cataract ) or lacrimal gland as well as disturbances in bone growth.
Brachytherapy
In brachytherapy, radiation carriers ( applicators ) are sewn onto the dermis ( sclera ) of the eye and removed again after the required radiation dose has been reached. The applicators contain small pieces of a radioactive emitter such as ruthenium-106 or iodine-125, the radiation of which can be aimed quite specifically at the tumor. It is therefore particularly suitable for medium-sized tumors away from the macula and localized vitreous seeds. Possible side effects are double vision , clouding of the lens , damage to the retina or the optic nerve, or bleeding.
Coagulation techniques
These are local therapy methods in which the tumor is killed by heating it with a laser ( laser coagulation ) or icing ( cryocoagulation ). They are used in patients with relatively early stages and small tumors, for relapses after radiation or after chemoreduction. The tumors must be removed from the optic nerve, macula , choroid and larger vessels so that they are not damaged by the treatment. Myopia , shrinkage of the dermis and damage to the retina can occur as complications .
chemotherapy
An adjuvant (supportive) chemotherapy is performed primarily by enucleation if a metastasis is present and tumor cells in the anterior chamber , iris or choroid were found in remote or optic nerve.
In chemoreduction , large tumors in the front part of the eye are made smaller by chemotherapy before they are treated further with local therapies (brachytherapy, laser or cryocoagulation).
A high-dose chemotherapy is performed only at high metastasis with poor prognosis.
Local application of chemotherapeutic agents directly into the ophthalmic artery ( ophthalmic artery ) is increasingly being used. This reduces systemic side effects.
In addition to the usual side effects of chemotherapy, the occurrence of chemotherapy resistance is problematic, as a result of which the tumor resumes growth after a while.
Thermochemotherapy
In the thermal chemotherapy is made of the effect is used that local heating of the tumor the effect of treatment with a chemotherapeutic agent (usually carboplatin significantly improved). It is mainly used for smaller tumors in the back of the eye. Possible complications are opacification of the vitreous humor and damage to the retina and iris.
New therapy methods
All current therapy methods are associated with a number of disadvantages: enucleation leads to the loss of an eye, percutaneous radiation therapy increases the risk of secondary tumors, chemotherapy is associated with considerable side effects and has only limited effectiveness due to the occurrence of resistance, laser coagulation and cryocoagulation result loss of retinal areas and thus impaired vision. Despite the low mortality , retinoblastoma is therefore associated with high morbidity . Novel therapy methods such as thermochemotherapy or photodynamic therapy are intended to further reduce morbidity.
history

The first description of a retinoblastoma is attributed to the Leiden anatomist Pieter Pauw , known as Petrus Parwius , who in 1597 in his “Observationes Anatomicae Selectiores” in the chapter “Tumor oculorum” describes the autopsy of a three-year-old boy who died of a large tumor of the left eye. In 1805, William Hey describes in detail in his “Practical Observations in Surgery” a “Fungus haematodes”, ie blood sponge. The most detailed investigation can be found in the "Observations on the Fungus Haematodes or Soft Cancer" by James Wardrop (1809), who describes 17 cases and describes for the first time the development in the retina and the spread via the optic nerve (N. opticus). He was the first to recommend removal of the eye. In 1836, Langenbech, Robin and Nystin were able to microscopically prove that it was formed in the retina. Rudolf Virchow referred to the tumor as the glioma retinae in 1864. In 1868 Albrecht von Graefe realized that it was a hereditary disease. Simon Flexner (1891) and Hugo Wintersteiner (1897) describe the typical rosette-shaped growth (Flexner-Wintersteiner rosettes) and recognize the relationship to rods and cones of the retina. The term "retinoblastoma" was coined by Fredrick Herman Verhoeff in 1926, who recognized the origin of the still undifferentiated retinal cells, the retinoblasts. In 1971 the retinoblastoma served as a model system for the “two-hit” hypothesis by Alfred G. Knudson , and in 1987 the “retinoblastoma susceptibility gene” was sequenced by Lee.
literature
- P. Pawius: Observatio XXIII. Tumor oculorum. In: Observationes Anatomicae Selectiores. Appended to: T. Bartholinus: Historiarum Anatomicarum Rariorum. Centuria III & IV. Petrus Morsing, Copenhagen, Denmark 1657, pp. 38-39. (Reprinted in: T. Kivelä, ML Polkunen: Pieter Pauw's Tumor Oculorum. Reappraisal of the Presumed First Description of Retinoblastoma in 1597. ) In: Arch Ophthalmol. 2003; 121,6: 881-886. PMID 12796262
- W. Hey: Practical Observations in Surgery: Illustrated by Cases. 1814. [1]
- S. Flexner: A peculiar glioma (neuroepithelioma?) Of the retina. In: Johns Hopkins Hosp Bull. 1891; 2, pp. 115-119.
- WH Lee, R. Bookstein, F. Hong, LJ Young, JY Shew, EY Lee: Human retinoblastoma susceptibility gene: cloning, identification, and sequence. In: Science . 1987 Mar 13; 235 (4794), pp. 1394-1399. (PDF) . PMID 3823889
- M. Chintagumpala, P. Chevez-Barrios, EA Paysse, SE Plon, R. Hurwitz: Retinoblastoma: review of current management. In: Oncologist . 2007 Oct; 12 (10), pp. 1237-1246. Review. PMID 17962617
- A. Melamud, R. Palekar, A. Singh. Retinoblastoma. At the Fam Physician. 2006 Mar 15; 73 (6), pp. 1039-1044. Review. PMID 16570739
- AG Knudson, Jr: Mutation and cancer: statistical study of retinoblastoma. In: Proc Natl Acad Sci USA . 1971 Apr; 68 (4), pp. 820-823. PMID 5279523
- Bornfeld among other things: Perspectives of Ophthalmooncology. In: Dtsch Arztebl. 2004; 101 (38), pp. A-2526 / B-2130 / C-2049.
Experience report of a person concerned:
- Becky Keep: eyes to see. Christliche Verlagsgesellschaft, Dillenburg 2018, ISBN
978-3-86353-486-8.
Individual evidence
- AH Berger, AG Knudson, PP Pandolfi: A continuum model for tumor suppression. In: Nature . 2011 Aug 10; 476 (7359), pp. 163-169. doi: 10.1038 / nature10275 . Review. PMID 21833082
- AL Manning, NJ Dyson: pRB, a tumor suppressor with a stabilizing presence. In: Trends Cell Biol . 2011 Aug; 21 (8), pp. 433-441. doi: 10.1016 / j.tcb.2011.05.003 . Epub 2011 Jun 12. Review. PMID 21664133
- SK Houston, TG Murray, SQ Wolfe, CE Fernandes: Current update on retinoblastoma. In: Int Ophthalmol Clin. 2011 Winter; 51 (1), pp. 77–91. Review. PMID 21139478
- D. Lohmann: Retinoblastoma. In: Adv Exp Med Biol. 2010; 685, pp. 220-227. Review. PMID 20687510
- CL Shields, JA Shields: Intra-arterial chemotherapy for retinoblastoma: the beginning of a long journey. In: Clin Experiment Ophthalmol. 2010 Aug; 38 (6), pp. 638-643. Epub 2010 Mar 25th Review. PMID 20584015
- CL Shields, JA Shields: Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. In: Curr Opin Ophthalmol. 2010 May; 21 (3), pp. 203-212. Review. PMID 20224400
- KE Nichols, S. Walther, E. Chao, C. Shields, A. Ganguly: Recent advances in retinoblastoma genetic research. In: Curr Opin Ophthalmol. 2009 Sep; 20 (5), pp. 351-355. Review. PMID 19587599
- V. Poulaki, S. Mukai: Retinoblastoma : Genetics and Pathology. In: Int Ophthalmol Clin. 2009 Winter; 49 (1), pp. 155-164. Review. No abstract available. PMID 19125074
- C. Rodriguez-Galindo, MW Wilson, G. Chantada, L. Fu, I. Qaddoumi, C. Antoneli, C. Leal-Leal, T. Sharma, M. Barnoya, S. Epelman, L. Pizzarello, JR Kane, R. Barfield, TE Merchant, LL Robison, AL Murphree, P. Chevez-Barrios, MA Dyer, J. O'Brien, RC Ribeiro, J. Hungerford, EM Helveston, BG Haik, J. Wilimas: Retinoblastoma : one world, one vision. In: Pediatrics. 2008 Sep; 122 (3), pp. E763 – e770. Review. PMID 18762512
Web links
- Retinoblastoma. In: Online Mendelian Inheritance in Man . (English)
- Diagnosis and therapy of retinoblastoma (PDF) (609 kB)
- Genetics of retinoblastoma (PDF) (596 kB)
- Children's Eye Cancer Foundation. Retrieved on June 19, 2010 (current information on disease, research and prevention).
- Retinoblastoma site of the German Cancer Society e. V.
- Retinoblastoma (brief information from July 19, 2011) on the website kinderkrebsinfo.de , accessed on February 24, 2014
- diagnosis
- Forms of therapy
- Initial examination