glaucoma
| Classification according to ICD-10 | |
|---|---|
| H40 | glaucoma |
| ICD-10 online (WHO version 2019) | |
The glaucoma , also Green Star called, is a set of eye diseases with different causes that have an irreversible damage to nerve fibers result. In the advanced course of the disease, this becomes noticeable at the exit point of the optic nerve as an increasing cavity ( excavation ) or pale and atrophy of the optic nerve head (papilla). As a result, characteristic visual field defects ( scotomas ) develop , which in extreme cases can lead to blindness in the affected eye . An elevated intraocular pressure (ocular hypertension) is a major risk factor for glaucoma. However, nearly 40 percent of all glaucoma patients have normal intraocular pressure ( normal tension glaucoma ), but are very sensitive to fluctuations in blood pressure, which makes an interdisciplinary, between ophthalmologists and internists coordinated treatment required.
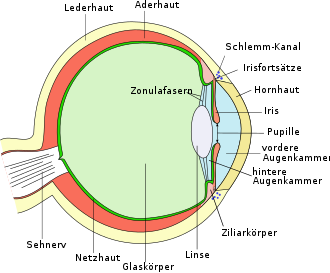
Open- angle and narrow-angle glaucoma can be differentiated according to anatomical criteria . These terms refer to the structure that the posterior surface of the cornea and the anterior surface of the iris form with each other as a so-called chamber angle . This is where the trabecular system is located , through which the aqueous humor flows out of the eye. Open-angle glaucomas are far more common and usually run chronically and unnoticed, while the rarer narrow-angle glaucomas can lead to painful glaucoma attacks that, if left untreated, lead to acute blindness within a short period of time. The visual field deficits in open-angle glaucoma often only become noticeable late because they start outside the center (peripheral) and can be covered by the intact visual field of the other eye.
Glaucoma is one of the most common causes of blindness worldwide. Around 500,000 Germans suffer from increased intraocular pressure, ten percent of whom are at risk of going blind. But even normal intraocular pressure does not rule out glaucoma. The professional association of ophthalmologists in Germany points out that the number of unreported cases in this area is very high. It is assumed that a total of around one million people in Germany are affected by glaucoma. At least there are signs of improvement. Between 1980 and 2000, the risk of going blind from glaucoma halved. Early detection and better treatment methods in particular are blamed for the decline.
Origin of name
The name glaucoma , coined by Aristotle , comes from the Greek γλαυκός glaukós = 'bright, shining, shiny' or, especially with regard to the sea, '[gray] bluish' and is derived from the blue-gray discoloration of the iris in chronic inflammation from. In the 16th century it became "green" or "sea-colored" in France, as in northern France the Atlantic looks more greenish than bluish.
Galenos understood glaucoma as a "yellowish or greenish-colored drying of the crystal" (ie the lens of the eye) caused by a reduction or thickening of the aqueous humor.
Star has actually been a term for lens opacities in German since the 8th century . In the 20th century, the term spread Green Star as a synonym for glaucoma. The glaucoma is not to be confused with the cataract , an opacification of the lens.
Emergence
The aqueous humor ( humor aquosus ) is a clear body fluid in the anterior and posterior chambers of the eye . It is produced in the ciliary body of the eye and delivered to the posterior chamber of the eye. It passes through the pupil into the anterior chamber of the eye and flows off in the chamber angle through the trabecular structure and Schlemm's canal . The intraocular pressure arises from the ratio of aqueous humor production to aqueous humor outflow, which is regulated by the resistance of the trabecular system.
Normal intraocular pressure is between 10 and 21 mm Hg . Fluctuations in the course of the day of up to 5 mm Hg are considered physiological , with the highest values usually occurring at night or in the early morning hours. Older people have, on average, higher intraocular pressure than younger people.
A disproportion between intraocular pressure and blood flow to the optic nerve is considered to be a mechanism that causes glaucoma. In the case of high blood pressure within the vessels of the optic nerve head, high intraocular pressure can be tolerated; in the case of low blood flow pressure, even low intraocular pressure can lead to the progression of glaucoma. The combination of high intraocular pressure and low blood pressure in the optic nerve head is particularly unfavorable. Since both intraocular pressure and perfusion pressure can fluctuate, both the absolute value of both parameters and the duration of phases of unfavorable pressure conditions are important. It should also be noted that the individual's sensitivity to pressure ( tension tolerance ) can play a major role in the development of glaucoma.
All conditions which on the one hand contribute to increased intraocular pressure or on the other hand to reduced perfusion pressure in the optic nerve head can cause glaucoma. The former include mainly disorders in the function of the trabecular system, the latter include arteriosclerosis and arterial hypotension . Combinations of several such factors are common, so that the critical eye pressure threshold for damage to the optic nerve head can vary from person to person. In addition to these development mechanisms, others are suspected because there are patients in whom glaucoma progresses despite normal intraocular pressure values and good blood flow to the optic nerve.
The damage to the optic nerve in glaucoma can be recognized by an inspection of the fundus ( funduscopy ) by a characteristic hollow (excavation) of the optic nerve head, affects first the nerve fibers of the middle retinal periphery and slowly progresses towards the center. About 70 percent of the nerve fibers affected, arcuate visual field defects form in the central visual field of (Bjerrum scotoma), which are themselves often not perceived in the advanced stage of the victims.
Typical of glaucoma is the creeping, but sometimes unpredictable, volatile course. For this reason, regular ophthalmological examinations are required in the event of suspicion or illness. Preventive examinations for glaucoma should also be carried out with a certain regularity.
Risk factors
Risk factors for developing glaucoma are:
- increased pressure in the eye
- enlarged excavation of the papilla (hollowing of the optic nerve head)
- small thickness of the central cornea
- Blood pressure deviating from the norm : On the one hand, very low blood pressure with intermittently low values favor the occurrence of glaucoma damage (this can also be the result of drug-treated blood pressure). On the other hand, too high blood pressure can also damage the vessels supplying the optic nerve. (see also normal pressure glaucoma)
- Diabetes mellitus can lead to secondary glaucoma.
- Genetic predisposition: If one or more close relatives are ill, there is a “familial” burden, one of the most important risk factors for glaucoma.
- Flammer syndrome : Circulatory problems ( vasospasm ) in the limbs (cold hands or feet, see also Raynaud's syndrome ), migraines or tinnitus , arterial hypotension (low blood pressure), a low body mass index , increased sensitivity to pain, or odors Medication, taking a longer time to fall asleep or a reduced feeling of thirst can be an indication of circulatory disorders of the optic nerve, which can lead to damage without increasing the intraocular pressure (normal pressure glaucoma).
- ethnic group : dark-skinned people are up to five times more likely to have glaucoma than fair-skinned people.
- high myopia (open-angle glaucoma)
- high farsightedness (narrow-angle glaucoma and glaucoma attack)
- old age
Diagnostic criteria
The eye pressure (tensio) is usually determined with a so-called applanation tonometer according to Goldmann . The force is measured that is required for a defined mechanical flattening of the cornea , which has previously been anesthetized with drugs . Alternatively, non-medical personnel can also carry out a non-contact measurement using pneumotonometry , a flattening of the cornea through a defined puff of air using a non-contact tonometer . Since the corneal thickness has an influence on the measured pressure values, an additional measurement of the corneal thickness ( pachymetry ) is recommended . Pressure values that fluctuate over the course of the day can be determined by several measurements distributed over the day (daily pressure profile).
Measuring the intraocular pressure alone is not sufficient either to establish a diagnosis or to rule it out, or to assess the progression of glaucoma. If only a pressure increase were used as a diagnostic criterion, half of the glaucomas would be overlooked. The following additional examinations are used:
- Assessment of the fundus in a three-dimensional image: The extent of the damage to the optic nerve is determined based on the size and shape of the papillary excavation (cavity of the optic nerve head). Defects in the nerve fiber layer can be found in the red-free light.
- Visual field examination (perimetry): Look for characteristic, arched defects ( scotomas ).
Newer imaging methods such as HRT (scanning laser tomography), RTA (retinal thickness measurement), GDx ( scanning laser polarimetry ), OCT ( optical coherence tomography ), record even minor damage in a reproducible and detailed manner and thus offer a significant diagnostic tool for early stage assessment and follow-up controls Support. In Germany, they represent IGeL services, the costs of which have not yet been covered by statutory health insurances.
Also is for classification of glaucoma an examination of the anterior segment with the slit lamp necessary to deposits of pigment on the corneal surface or pathological protein on the lens or the Irissaum ( pseudoexfoliation ), a study on newly iris vessels ( rubeosis iridis ), corneal opacities (embryotoxon or Haab lines) and pigment defects in the iris (church window phenomenon). Furthermore, with the help of a contact glass, the width (narrow-angle glaucoma or chamber-angle recess) and morphology (anomalies) of the chamber angle can be assessed ( gonioscopy ).
The light tail test (also: Bagolini test ) can be used for a rough exposure examination of visual field deficits , especially in glaucoma.
The collective term glaucoma and the different forms of glaucoma
The ICD chapter H40.- "Glaucoma" lists not only the various forms in which the disease glaucoma occurs, but also " Ocular hypertension " (H40.0). That is why it is listed here.
Ocular hypertension
The "ocular hypertension" (English: ocular hypertension, abbreviation: OHT) describes a condition of increased intraocular pressure without any pathological damage to the eye having occurred at the time of the examination. Ocular hypertension often develops into the disease "primary open-angle glaucoma" within a few years with damage to the nerve fibers of the retina or the optic nerves. The higher the measured intraocular pressure, the higher the risk of suffering the damage. The ocular hypertension therefore counts as a preliminary stage or possible early stage of glaucoma and is treated as a precautionary measure to lower intraocular pressure, if necessary (not in all cases) after weighing the risk / benefit ratio. According to the results of the "Ocular Hypertension Treatment Study", the preventive lowering of intraocular pressure significantly reduces the risk of damage.
Open-angle glaucoma
Primary open-angle glaucoma
Primary open-angle glaucomas (POAG) are open-angle glaucomas that do not occur as a result of another eye disease.
Glaucoma chronicum simplex
synonymous: primary chronic glaucoma
It is the most common type of glaucoma. It typically occurs from the age of 40, but it can also start earlier. Familial accumulation, i.e. a disposition-related (genetic) component, is known. With glaucoma chronicum simplex, there is an obstruction to drainage directly in the drainage area of the chamber angle due to degenerative changes. The pressure inside the eye increases slowly over the years and the person concerned usually does not feel any discomfort.
Another form of glaucoma chronicum simplex is normal pressure glaucoma , also incorrectly referred to as low pressure glaucoma. In this case, progressive damage to the optic nerve occurs despite predominantly normal intraocular pressure values. The local blood flow to the optic nerve head is restricted by various factors, whereby the optic nerve fibers are also damaged. It is estimated that around 40 percent of all glaucomas are normal pressure glaucomas, which require interdisciplinary treatment by an ophthalmologist and internist, and which are more the clinical picture of a vascular neuropathy than damage to the optic nerve from mechanical influences.
A thin cornea can underestimate intraocular pressure in applanation tonometry by up to 3 mm Hg, which in some cases may have led to an unjustified classification as normal pressure glaucoma. It is also discussed whether a thin cornea is an independent risk factor for glaucoma.
Congenital glaucoma
synonymous: juvenile glaucoma, congenital glaucoma
Developmental disorders of the chamber angle during the embryonic period lead to a disturbance of the drainage of the aqueous humor. This can occur in combination with other malformations of the body. A common cause is rubella infection in early pregnancy. The increased intraocular pressure can lead to unilateral or bilateral enlargement of the eyeball Hydrophthalmus (Buphthalmus). Congenital glaucoma should be considered if the cornea is enlarged, the cornea is cloudy and is photophobic. An examination, intraocular pressure measurement and, if necessary, surgery under anesthesia must be carried out at an early stage to prevent permanent visual impairment.
Secondary open-angle glaucoma
If open-angle glaucoma is caused by other eye diseases, it is called secondary open-angle glaucoma. This is the case with injuries or inflammations of the eye ( uveitis ), intraocular tumors , with neovascularization in the chamber angle, for example as a result of diabetes mellitus, or the use of certain drugs (e.g. cortisone in steroid responders ) in people with a predisposition .
Pseudoexfoliative glaucoma
A special, but frequently occurring form of secondary glaucoma represents the pseudoexfoliation (short PEX glaucoma; also Pseudoexfoliation ) is Here it comes through fine fibrillar deposits on the lens and in the chamber angle to barriers to the outflow of aqueous humor and due to some massive pressure increases.. The composition of the deposit material has not yet been fully clarified. However, it has been proven that it can be formed on the one hand by the lens epithelium and on the other hand not only in the eye, but also in other organs of the body such as the heart , lungs , liver , kidneys or gall bladder .
Pigmentary glaucoma
In predisposed eyes, pigment abrasion from the back surface of the iris through the lens or zonular fibers can lead to an accumulation of pigment (or melanin granules) in the trabecular structure of the chamber angle (pigment dispersion). This can lead to a chronic increase in pressure. In addition, the contact between the iris and the lens can lead to an "inverse" pupillary block, so that the pressure in front of the iris (anterior chamber) is greater than that behind it (posterior chamber). This causes - like a cycle of errors - further contact between the iris and the lens with increased pigment abrasion.
Usually this constellation affects people in the 3rd-5th centuries. Decade of life, often associated with low myopia. Typical findings in the slit lamp examination are clearly visible pigment deposits on the back surface of the cornea ( Krukenberg spindle), a deep anterior chamber and defects in the iris pigment epithelium, which can be easily recognized as church window phenomena in the falling light. Pigment (melanin) can also be detected on lens fibers and gonioscopy in the chamber angle.
Angle-closure glaucoma
Narrow-angle glaucoma is caused by an impaired drainage of the aqueous humor as a result of a narrow point between the iris (iris) and the cornea in front of the trabecular system (in the chamber angle). The extent of the constriction can vary due to the change in pupil size and thus the thickness of the iris. The drainage disorder periodically or constantly increases intraocular pressure, which ultimately leads to optic nerve damage. People with greater farsightedness (because of the relatively acute chamber angle) and advanced cataracts (because of the thick lens of the eye) are more prone to narrow-angle glaucoma. Pupil dilatants, anticholinergic drugs such as some antidepressants or antiemetics can cause an increase in intraocular pressure via this mechanism and lead to glaucoma attacks.
Glaucoma attack
synonymous: Glaucoma acutum
The acute attack of glaucoma is based on a sudden decrease in the drainage of the aqueous humor due to an obstruction of the chamber angle by the iris (angle block). The acute angle block leads to a drastic increase in pressure up to more than three times the normal value (70 mm Hg) and palpable ( palpatory ) rock-hard eyeball. Symptoms of the glaucoma attack come on suddenly and range from reddened eyes to eye pain to gastrointestinal symptoms such as nausea and vomiting. Corneal edema can cause a sudden deterioration in visual acuity in the affected eye. Accompanying the increase in pressure, severe headaches, sometimes with cardiac arrhythmias and the perception of colored rings in the backlight, are possible. The pupil is of medium size and often does not react or only hardly reacts to light radiation. Usually only one eye is affected. The seizure can spontaneously subside after a few hours and recur at intervals, but it can also last for days without being recognized. Every acute angular block is an emergency that requires immediate therapy. A prophylactic operation may have to be performed on the partner's eye, which is often also at risk of angle-closure glaucoma due to comparable anatomical conditions.
treatment
If damage to the optic nerve typical of glaucoma is found, the intraocular pressure must be reduced permanently. Damage to the optic nerve usually sets in when a critical intraocular pressure is chronically exceeded. This critical pressure varies from person to person (target pressure) and must first be found individually in the course of the disease through close-knit checks and then undercut as permanently as possible through appropriate treatment (usually around 15 Torr ). The aim of therapy is therefore to prevent the disease from progressing, damage that has occurred ( visual field defects ) cannot be reversed.
The European Glaucoma Society has issued therapy guidelines. The first step in glaucoma therapy is local medication with eye drops; initially as monotherapy, later as combination therapy. If the target pressure can no longer be achieved below this, surgical procedures follow, usually initially the so-called trabeculectomy , which is still considered the gold standard in glaucoma surgery. As an alternative to drug monotherapy, laser trabeculoplasty (using argon laser - ALT - or selective laser trabeculoplasty using SLT) can initially be considered.
Furthermore, internal treatment is indicated in many cases. There are also studies that indicate that relaxation exercises such as autogenic training help to lower intraocular pressure and can therefore be used in conjunction with therapy. Acupuncture treatment has no effect on intraocular pressure.
Medical therapy
Various substances are available for drug therapy of glaucoma, most of which are administered as eye drops:
- Beta blockers : timolol , levobunolol
- Cholinergics : carbachol , pilocarpine
- Alpha-2 adrenoceptor agonists: clonidine , brimonidine
- Carbonic anhydrase inhibitors : locally as eye drops as brinzolamide and dorzolamide or systemically in tablet form as acetazolamide
- Prostaglandins : latanoprost , travoprost , bimatoprost, tafluprost
Mode of action:
- Decrease in the production of aqueous humor from the ciliary body : beta blockers , alpha sympathomimetics , carbonic anhydrase inhibitors
- The prostaglandins increase the permeability of the ciliary body, and the so-called non-conventional drainage or uveoscleral outflow is increased.
- Cholinergics work by contracting the ciliary body, which opens the trabecular network. The additional pupil-constricting effect when the chamber angle is opened is an advantage in narrow-angle glaucoma.
The above drugs can also be combined. Combination preparations are also available for easier application . Usually it is a lifelong therapy. In the case of secondary glaucoma, treatment of the underlying disease may also be necessary.
Laser surgery
- Laser cyclodestruction / cyclophotocoagulation: obliteration of the ciliary body , which forms the aqueous humor, and at the same time the formation of scars through which the aqueous humor can drain. The procedure can be performed externally or endoscopically inside the eyeball. Last possibility of pressure reduction after failure of other interventions.
- Argon laser trabeculoplasty: Improvement of the outflow through laser application at the chamber angle , results in scarring and can therefore only be applied 1–2 times.
- Selective laser trabeculoplasty : Improvement of the aqueous humor outflow through selective laser application at the chamber angle, no tissue damage and therefore repeatable, is discussed as an initial measure before the administration of medication.
- Neodymium-YAG laser iridotomy : Improvement of the passage of aqueous humor from the posterior chamber into the anterior chamber by creating an opening in the outer iris (see also iridectomy ), only for a special form of glaucoma with a narrow chamber angle.
Operations
The following procedures are available:
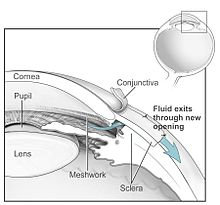
- Goniotrepanation and trabeculectomy: On the dermis (sclera), an outflow fistula is created from the anterior chamber of the eye under the conjunctiva . In certain patients it can make sense that the fistula only extends as far as Schlemm's canal and not into the anterior chamber (visco-canal ostomy).
- Cyclocoagulation: obliteration of the ciliary body by laser (see above) or cold probe (transconjunctival cyclocryocoagulation).
- Trabeculotomy and goniotomy: In dysgenic (congenital) glaucoma, the trabecular meshwork is opened and the Schlemm's canal is connected to the anterior chamber of the eye so that the aqueous humor can drain away again.
- Iridectomy: opening the iris in a narrow-angle situation. By making a small opening in the periphery of the iris, a pressure equalization between the anterior and posterior chambers is brought about, which is able to lift the angular block and thereby normalize the intraocular pressure. (see also laser iridotomy)
- Canaloplasty: Very new procedure (since the mid-2000s) in which a ring-shaped implant is placed through Schlemm's canal, which remains permanently and keeps the canal open.
- Implants: Micro-invasive glaucoma surgery (MIGS) improves the natural drainage pathways of the aqueous humor in the eye. The smallest such implant, the iStent made of titanium, is designed to keep such channels open permanently. Since, unlike in trabeculectomy, there is no opening to the outside, the complication rate is very low; the iStent and other implants are particularly recommended for moderate clinical pictures. In a study group of 192 patients, one year after implantation of an iStent, almost 95 percent of the patients had an intraocular pressure that was 20 percent or more lower than the preoperative value, and this without using eye drops to lower the pressure. The reduction in intraocular pressure by the iStent is described as being at least as pronounced as if the patient were using two different medications.
In addition, intraocular pressure can drop after cataract surgery . By removing the often voluminous lens, which pushes the iris forward, the anterior chamber deepens and the angle of the chamber is widened.
Prevention / early detection
There is no preventive measure to avoid illness. Early detection is the only way to prevent glaucoma damage with manifest restrictions of the field of vision as well as visual impairment and even blindness. Since the symptoms of the disease are noticed very late by the patient due to the compensation mechanisms of the brain and the damage is then irreversible, regular examinations by the ophthalmologist are necessary for early detection. The aim is to detect the disease before it becomes functional in its early stages. Timely treatment can prevent progression and ultimately blindness in most cases.
The economic value of screening healthy individuals is controversial. The glaucoma examination is usually only a service of the statutory health insurance if there are suspicions for an illness or an increased intraocular pressure is already known. In 2004, the Federal Joint Committee (GBA) refused to reinstate glaucoma screening in the catalog of services of the statutory health insurance companies. Components of a glaucoma screening are the intraocular pressure measurement ( tonometry ), the assessment of the fundus ( funduscopy ) and the visual field measurement ( perimetry ). An exclusive intraocular pressure measurement, as offered by opticians and many ophthalmologists, is not sufficient to assess whether glaucoma is present, since there are also glaucomas that show normal intraocular pressure, so-called normal pressure glaucoma . In January 2012 , the IGeL monitor of the MDS (Medical Service of the Central Association of Health Insurance Funds) rated the measurement of intraocular pressure alone for glaucoma early detection as "generally negative". In February 2015, the IGeL-Monitor also rated the ophthaloscopy with measurement of intraocular pressure for glaucoma early detection as "tending to be negative", because insufficient data means that it is not possible to assess who is receiving correct or incorrect test results (no indications of benefit, but indications of harm ).
The German Ophthalmological Society contradicted this in August 2015 and described the scientific findings on the benefits of early detection and treatment in a statement: “From a scientific point of view, population studies and prospective randomized therapy studies have proven two kinds of“ evidence-based ”methods in the last few decades: 1. Targeted Ophthalmological examinations can detect previously undetected glaucomas. 2. The therapeutic lowering of the intraocular pressure can stop the progression of the glaucoma disease and therefore save eyesight. ”The IGeL monitor is based on a report by the US agency for Healthcare Research and Quality (AHRQ), the benefits and harms of Preventive and early diagnosis examinations analyzed. Result of the report "Screening for Glaucoma: Comparative Effectiveness": Overall, no statement can be made from the scientific literature as to whether a combination of the two procedures for prevention and early detection can actually prevent glaucoma or blindness caused by glaucoma in later years. Nor have studies been found that investigated the question of how well a combination of the two methods can detect glaucoma at an early stage.
research
A microsensor called Eyemate , which received CE approval in 2017 and was successfully implanted in a number of selected patients as part of a study at the University Eye Clinic in Aachen, is intended to enable continuous measurement over 24 hours and over long periods of time . The intraocular pressure values are transmitted from the chip positioned in the anterior chamber to a reading device held by the patient at eye level and then telemetrically transmitted to the treating ophthalmologist. This method promises better control of the massive pressure fluctuations that frequently occur over the day and night.
See also
Sources and literature
- European Glaucoma Society: Terminology and guidelines for glaucoma. 4th edition. Edited by the European Glaucoma Society. PubliComm, Savona - Italy 2015, ISBN 978-88-98320-14-1 .
- Katarzyna Konieczka, Konstantin Gugleta, Ronald D. Gerste: Glaucoma, a manual for those affected, an introduction for those interested, a reference work for those in a hurry. Founded by Josef Flammer . 4th, revised edition. Huber, Bern 2015, ISBN 978-3-456-85146-4 .
- Thomas S. Dietlein, Manuel H. Hermann, Jens F. Jordan: Drug and surgical therapy of glaucoma. In: Deutsches Ärzteblatt . Volume 106, No. 37, 2009, pp. 597-606. doi: 10.3238 / arztebl.2009.0597 .
- Norbert Pfeiffer: Glaucoma and Ocular Hypertension. Basics, diagnostics, therapy. 2nd Edition. Thieme, Stuttgart et al. 2005, ISBN 3-13-105852-8 .
- Robert L. Stamper, Marc F. Lieberman, Michael V. Drake: Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. 8th edition. Mosby Elsevier, Edinburgh 2009, ISBN 978-0-323-02394-8 .
- Stefan Uhrig: Acupuncture treatment of glaucoma and ocular hypertension - basics of Chinese medicine and results of a prospective observational study. In: Chinese Medicine. 18, 2003, pp. 139-147.
- Guideline No. 15a from BVA and DOG : primary chronic open-angle glaucoma, normal pressure glaucoma and ocular hypertension.
- Franz Grehn : Ophthalmology. 30th edition. Springer, Berlin 2008, ISBN 978-3-540-75264-6 .
- Johann Peter Engels: The normal pressure glaucoma: results of the goniotrepanation according to Fronimopoulos in patients with normal pressure glaucoma in the years 1984–1988 (= Deutsche Hochschulschriften. Volume 2396). Hänsel-Hohenhausen, Egelsbach / Frankfurt am Main / St. Peter Port 1996, ISBN 3-8267-2396-1 . (Dissertation University of Witten, Herdecke 1996)
Web links
- European Glaucoma Society website
- www.bundesverband-auge.de - Federal Association of Eye eV
- Article in the NDR about glaucoma and modern surgical therapy (MIGS) ndr.de
- Glaucoma patient information and guidelines (PDF; 174 kB) from the Professional Association of Ophthalmologists (BVA) and the German Ophthalmological Society (DOG)
- Article in Die Welt am Sonntag of December 27, 2015.
- "The patient often only notices something about glaucoma when it is already too late." - Interview with chief physician Dr. Lösche, Mülheim an der Ruhr
- www.glaukom.de - Initiativkreis Glaucoma Early Detection e. V.
- www.glaucoma-association.com - International Glaucoma Society (IGA)
- www.glaukompatienten.ch - Glaucoma group of the Swiss Ophthalmological Society
Individual evidence
- ↑ a b Ronald D. Barley: Glaucoma: A Vascular Neuropathy. In: Deutsches Ärzteblatt. 105 (11), 2008, pp. A-562 / B-500 / C-489.
- ↑ Mehrdad Malihi, Edney R. Moura Filho, David O. Hodge, Arthur J. Sit: Long-Term Trends in Glaucoma-Related Blindness in Olmsted County, Minnesota. In: Ophthalmology. 121, 2014, pp. 134-141. doi: 10.1016 / j.ophtha.2013.09.003 .
- ^ Wilhelm Gemoll : Greek-German school and hand dictionary. Reviewed and expanded by Karl Vretska . 9th edition. Freytag et al., Munich et al. 1965. (Reprint: Hölder-Pichler-Tempsky, Vienna 1997, ISBN 3-209-00108-1 ).
- ↑ Frank Krogmann: Star, greener (glaucoma). In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. de Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , pp. 1355 f .; here: p. 1355.
- ^ Franz Grehn: Ophthalmology. Springer Verlag, 2013, ISBN 978-3-662-05918-0 , p. 334.
- ↑ Katarzyna Konieczka et al: Flammer syndrome. In: The EPMA Journal. 5, 2014, p. 11.
- ↑ a b Information on glaucoma from the Professional Association of Ophthalmologists in Germany (BVA) and the German Ophthalmological Society (DOG) (PDF; 121 kB)
- ↑ M. Pache, J. Funk: High-tech in glaucoma diagnostics. In: Clinical monthly sheets for ophthalmology. Volume 223, No. 6, 2006, ISSN 0023-2165 , pp. 503-508. doi: 10.1055 / s-2005-859004 .
- ↑ AAD - Ophthalmological Academy of Germany ( Memento of the original from December 16, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ The strip glasses according to Bagolini for the detection of visual field defects in glaucoma. 97th Annual Meeting of the German Ophthalmological Society (DOG), 1999.
- ^ Ocular Hypertension Treatment Study (OHTS) - Full Text View - ClinicalTrials.gov. Retrieved May 16, 2017 (English).
- ↑ Study examines effects of delaying treatment for ocular hypertension. Science Daily, March 8, 2010, accessed May 22, 2017 .
- ↑ Keith L. Moore, T. Vidhya N. Persaud: Embryology: stages of development, early development, organogenesis, clinic. Translated and edited by Christoph Viebahn. 5th edition. Elsevier, Urban & Fischer, Munich et al. 2007, ISBN 978-3-437-41112-0 , p. 520.
- ↑ Torsten Schlote, Jens Martin Rohrbach (ed.): Secondary glaucoma. Complicated glaucoma in theory and practice. Schattauer, Stuttgart 2004, ISBN 3-7945-2266-4 .
- ↑ K. Rigal: Pseudoexfoliations- und Pigmentglaucoma. In: Spectrum of Ophthalmology. Volume 18, No. 5, 2004, ISSN 0930-4282 }, pp. 248-250. doi: 10.1007 / BF03163179 .
- ^ Dietmar Kühn, Jürgen Luxem, Klaus Runggaldier: Ambulance service . 3. Edition. Elsevier, Urban & Fischer, Munich et al. 2004, ISBN 3-437-46191-5 .
- ↑ European Glaucoma Society: Terminology and action guidelines for glaucoma . Ed .: European Glaucoma Society. 4th edition. PubliComm, Savona - Italy 2015, ISBN 978-88-98320-14-1 .
- ↑ Relieve the pressure on the eyes: Autogenic training and the like help with glaucoma. on: Medizin-aspekte.de
- ↑ Wolfgang Leydhecker: Advances in modern ophthalmology. In: Würzburg medical history reports. Volume 3, 1985, pp. 198 and 209.
- ↑ Anna Julia Voormann: New therapy for glaucoma: mini implants as an alternative to eye drops and surgery. German Ophthalmological Society , press release from September 22, 2016 at Informationsdienst Wissenschaft (idw-online.de), accessed on September 23, 2016.
- ↑ Thomas W Samuelson: Microinvasive glaucoma surgery - Coming of age. In: Journal of Cataract and Refractive Surgery . 40, August 2014, pp. 1253-1254.
- ↑ Antonio M. Fea et al .: Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. In: Clinical Ophthalmology. 14, August 2014, pp. 875-882.
- ↑ European Glaucoma Society (Ed.): Terminology and Guidelines for Glaucoma. 2nd Edition. Editrice Dogma, Savona 2003, ISBN 88-87434-13-1 .
- ↑ Press release of the Federal Joint Committee v. 2005
- ↑ Professional Association of Ophthalmologists in Germany 2012
- ^ Professional Association of Ophthalmologists (BVA), press release of February 2, 2012.
- ↑ IGeL-Monitor, evaluation of the ophthalmoscope with measurement of the intraocular pressure for the early detection of glaucoma , accessed on November 8, 2018.
- ^ Statement by the German Ophthalmological Society , November 6, 2015.
- ↑ IGeL monitor, evidence synthesis and result report of the evaluation of the ophthalmoscope with intraocular pressure measurement.
- ↑ A. Koutsonas, P. Walter, G. Roessler, N. Plange: Long-term follow-up after implantation of a telemtric intraocular pressure sensor in patients with glaucoma: a safety report. In: Clin Exp Ophthalmol. 2017, published online on November 14th. doi: 10.1111 / ceo.13100in .
- ↑ Ronald D. Gerste: Glaucoma: Miniaturization in diagnostics and therapy. In: Deutsches Ärzteblatt. 113 (39), 2016, pp. A-1710 / B-1446 / C-1422.