Latanoprost
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Latanoprost | ||||||||||||||||||
| other names |
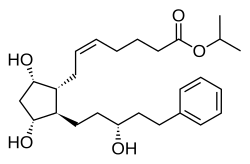
Isopropyl ( Z ) -7 - [(1 R , 2 R , 3 R , 5 S ) -3,5-dihydroxy-2 - [(3 R ) 3-hydroxy-5-phenylpentyl] -cyclopentyl] hept-5- enoat |
||||||||||||||||||
| Molecular formula | C 26 H 40 O 5 | ||||||||||||||||||
| Brief description |
colorless oil |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 432.29 g · mol -1 | ||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Latanoprost is a medicine that is used in the topical treatment of glaucoma and increased intraocular pressure . To do this, it is used in the form of eye drops. Latanoprost promotes the drainage of aqueous humor through the vessels of the ciliary body and the iris (uveoscleral or uveovortical drainage), which reduces the increased pressure.
The substance belonging to the group of prostaglandins is a colorless, oily liquid.
Latanoprost is listed on the World Health Organization's list of essential medicines .
Latanoprost is an isopropyl ester prodrug . This means that it is inactive until it is hydrolyzed by esterases in the cornea . It was developed by Johan W. Stjernschantz and Bahram Resul as employees of the Pharmacia company.
Medical use
Increased eye pressure
- In clinical studies in patients with open-angle glaucoma or increased intraocular pressure (≥21 mmHg), latanoprost was found to decrease pressure by 22% to 39% after treatment for 1 to 12 months. Latanoprost was significantly more effective than timolol and showed a time-stable effect of lowering pressure.
- Meta-studies show that latanoprost is more effective than timolol at lowering eye pressure, but it often causes iris pigmentation. This appears to be benign , but patients should be examined for this long-term.
Angle-closure glaucoma
- Latanoprost was also more effective than timolol in patients with narrow-angle glaucoma .
Latanoprost is effective at doses of 1.56 micrograms per day. A higher dosage reduces the effect of lowering pressure in the eye.
Side effects
List from common to very rare:
- > 5–15%: Blurred vision, burning or stinging sensation, conjunctival hyperemia , sensation of foreign bodies, itching, increased pigmentation of the iris, which can lead to iris heterochromia , superficial punctate keratitis
- 4%: Cold or upper respiratory tract infection, flu-like symptoms
- 1–4%: dry eyes, strong tear formation, eye pain, crusting of the eyelid, eyelid edema , erythema of the eyelid (hyperemia), eyelid pain, sensitivity to light
- 1–2%: chest pain, allergic skin reactions, arthralgia , back pain, muscle pain , thickening of the eyelashes
- <1% (only important or life-threatening): asthma, herpes keratitis , iritis, keratitis, retinal artery embolism , detachment of the retina , Lyell syndrome , uveitis , vitreous hemorrhage due to diabetic retinopathy
Contraindications and interactions
If you are hypersensitive to latanoprost, the drug must not be used. If the following drugs are administered at the same time, interactions may occur:
- Bimatoprost : The simultaneous administration of latanoprost and bimatoprost can lead to higher intraocular pressure.
- Nonsteroidal anti-inflammatory drugs : These can either weaken or strengthen the therapeutic (ophthalmic) effect of prostaglandins.
storage
Latanoprost is thermally and especially unstable to UV radiation. For example, the concentration of latanoprost decreases by 10% every 8.25 days when it is stored at 50 ° C. The recommended storage is therefore protected from light at −20 ° C.
Finished medicinal products
Xalatan, ( Pfizer ), Xalacom; The patent for latanoprost expired in March 2011, so generics have been available since then .
Individual evidence
- ↑ Entry on latanoprost. In: Römpp Online . Georg Thieme Verlag, accessed on November 15, 2014.
- ↑ a b Entry on latanoprost in Pharmawiki , accessed on January 2, 2015.
- ↑ Latanoprost data sheet at Tocris, accessed on November 15, 2014.
- ↑ a b c Datasheet Latanoprost from Sigma-Aldrich , accessed on November 15, 2014 ( PDF ).
- ↑ a b S. S. Patel, CM Spencer: Latanoprost. A review of its pharmacological properties, clinical efficacy and tolerability in the management of primary open-angle glaucoma and ocular hypertension . In: Drugs Aging . 9, No. 5, 1996, pp. 363-378. doi : 10.2165 / 00002512-199609050-00007 . PMID 8922563 .
- ^ Kristiina M. Huttunen, Hannu Raunio, Jarkko Rautio: Prodrugs — from Serendipity to Rational Design . In: Pharmacological Reviews . tape 63 , no. 3 , January 9, 2011, p. 750-771 , doi : 10.1124 / pr.110.003459 , PMID 21737530 .
- ↑ Patent US5296504 : Prostaglandin derivatives for the treatment of glaucoma or ocular hypertension. Published March 22, 1994 .
- ↑ CM Perry, JK McGavin, CR Culy, T. Ibbotson: Latanoprost. An Update of its Use in Glaucoma and Ocular Hypertension . In: Drugs Aging . 20, No. 8, 2003, pp. 1170-2229. PMID 12795627 .
- ↑ WY Zhang, AL Wan Po, HS Dua, A. Azuara-Blanco: Meta-analysis of randomized controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension . In: British Journal of Ophthalmology . 85, 2001, pp. 983-990. doi : 10.1136 / bjo.85.8.983 . PMID 11466259 . PMC 1724079 (free full text).
- ↑ T. Aung, HT Wong, CC Yip, JY Leong, YH Chan, PT Chew: Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: a preliminary study. In: Ophthalmology. Volume 107, Number 6, June 2000, pp. 1178-1183, doi: 10.1016 / s0161-6420 (00) 00073-7 , PMID 10857840 .
- ^ Parham V. Morgan, Stefan Proniuk, James Blanchard, Robert J. Noecker: Effect of temperature and light on the stability of latanoprost and its clinical relevance . In: Journal of glaucoma . tape 10 , no. 5 , 2001, p. 401-405 .