Prodrug
As a prodrug ( the or the prodrug), an inactive or less active is a pharmacological substance referred to, the first by metabolism ( metabolism ) in the organism into an active drug ( metabolite is transferred).
Prodrugs are of strategic importance in those cases in which the actually active agent, if administered directly, does not reach the desired site of action, or only slightly or not selectively enough. The prodrug concept is primarily aimed at improving the pharmacokinetic properties of the substance. For example, the use of prodrugs can improve oral absorption or bioavailability , reduce the first-pass effect , or enable a drug to cross the blood-brain barrier .
A co-drug (mutual prodrug) is a special form of prodrug. It is a drug that is converted into two or more active ingredients in the body. Examples are fenetylline and sulfasalazine .
Examples
An example of a prodrug is levodopa , which is the starting material for the body's own synthesis of adrenaline , noradrenaline and dopamine . Levodopa is used as a prodrug for the treatment of Parkinson's disease : after crossing the blood-brain barrier , levodopa is metabolized into dopamine, which then develops the pharmacological effectiveness that is actually desired.
Further examples
- Codeine and diamorphine - active metabolite: morphine
- Clopidogrel - an inhibitor of platelet aggregation
- Metamizole - active metabolite: 4-N-methylaminoantipyrine (MAA)
- Myristicin - conversion into the hallucinogenic and euphoric 3-methoxy-4,5-methylenedioxyamphetamine (MMDA)
- Nabumetone - Non-steroidal anti-inflammatory drug
- Omeprazole - stomach acid inhibitor
- Phenacetin - active metabolite: paracetamol
- Ramipril and enalapril - active metabolites: ramiprilat and enalaprilat , drugs of the group of ACE inhibitors
- Lisdexamfetamine - active metabolite: dextroamphetamine
- Amfepramone - active metabolite: ethcathinone
- Adrafinil - active metabolite: Modafinil
- Psilocybin - active metabolite: psilocin
Co-drugs
- Fenetylline - effective metabolites: amphetamine and theophylline
- Sulfasalazine - effective metabolites: sulfapyridine and 5-ASA
Resorption ester
The so-called resorption esters form their own form of prodrugs . It is understood to mean compounds that have been synthetically esterified specifically for the purpose of being able to be absorbed better or at all. The esterification improves the lipophilicity and increases the absorption of the drug through the intestinal mucosa and thus its oral bioavailability. The ineffective or ineffective resorption ester is hydrolyzed either as soon as it passes through the intestinal mucosa or later in the plasma by the body's own esterases , with the pharmacologically active parent substance being produced again .
For example, the virostatic substance aciclovir is converted into the resorption ester valaciclovir through esterification of its hydroxyl group with the amino acid valine . The fosamprenavir is a Resorptionsester of amprenavir with phosphoric acid . Occasionally, steroid hormones are also esterified with simple carboxylic acids such as acetic acid , valeric acid or pivalic acid to form better absorbable derivatives, such as. B. fludricortisone -21-acetate, cortisone -21-acetate, abiraterone acetate , estradiol -17-valerate, prednisolone -21-pivalate, dexamethasone -21-pivalate, etc.
Certain active ingredients with carboxy groups can be converted to easily resorbable double esters of the acyloxyalkyl ester or alkoxycarbonyloxyalkyl ester type . This principle is z. B. used in oral cephalosporins and antivirals . The names of such resorption esters end in -xil or -xetil . The affix in front of it, such as B. -pi- , -a- or -pro- represents the acid used.
- Examples of cephalosporins: Cefotiamcilexetil , Cefuroximpivoxetil , Cefuroximaxetil, Cefpodoxime proxetil , Cefedametpivoxil .
- Examples of antivirals: tenofovir disoproxil , adefovir dipivoxil
Candesartan cilexetil is the absorption ester of the AT 1 antagonist candesartan .
ProTides
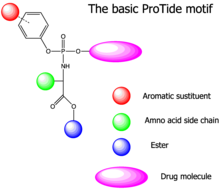
A prodrug concept developed by McGuigan and his team at Cardiff University are the aryloxyphosphoramidate prodrugs (“ProTide”, pro drug nucleo tide ). These are nucleotides or nucleotide analogues whose phosphate or phosphonate group is replaced by an aryl substituent and an amino acid ester is masked. This facilitates the passive diffusion of the substance through the cell membrane to its site of action. Nucleotide analogs are typical active ingredients in antiviral therapy . The preferred amino acid motif for a “ProTide” is L-alanine , the ester component is formed by short linear ( methyl , ethyl , pentyl ) or branched alkyl ( isopropyl , neopentyl ) and benzyl groups . As aryl moiety typically be phenyl and 1-naphthyl used. After absorption into the interior of the cell, the substituents are enzymatically split off in several steps and the nucleotide is released, which is further phosphorylated to the corresponding biologically active di- and triphosphate forms .
Examples of Aryloxyphosphoramidat prodrugs are about Tenofoviralafenamid , sofosbuvir and Remdesivir .
Individual evidence
- ↑ Prodrug . In: Duden - The large foreign dictionary: Origin and meaning of foreign words (online). 4th updated edition. Dudenverlag, Mannheim a. a. 2007.
- ↑ a b Prodrugs - drugs with tailor-made properties. In: Pharmazeutische Zeitung , issue 26/2011.
- ↑ M. Slusarczyk et al .: Phosphoramidates and phosphonamidates (ProTides) with antiviral activity . In: Antiviral Chemistry and Chemotherapy . tape 26 (2018) , pp. 1-31 , doi : 10.1177 / 2040206618775243 .
- ↑ Y. Mehellou et al .: The prodrug protide Technology: From the concept to the Clinic . In: Journal of Medical Chemistry . tape 61 (2018) , pp. 2211-2266 , doi : 10.1021 / acs.jmedchem.7b00734 .