Acyclovir
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
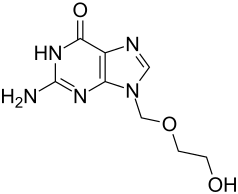
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Acyclovir | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
white crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 225.21 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
256.5-257 ° C |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Acyclovir is a drug from the group of antivirals . It is used to treat infectious diseases caused by certain viruses in the herpes virus family .
Chemically, acyclovir is a derivative of the nucleobase guanine , which occurs as a component of DNA and RNA .
Presentation and extraction
Various synthesis variants have been described for acyclovir . All variants are based on guanine, with protective groups being introduced in the first step by reaction with acetic anhydride or hexamethyldisilazane . The structural element of the hydroxyethoxymethyl groups can be generated by the reaction of 1,3-dioxolane with acetic anhydride. The resulting 2-acetoxyethylacetoxymethyl ether is then reacted with the diacetylguanine. After basic removal of the protective groups, the target compound is obtained.
application areas
Aciclovir is used for infections with herpes simplex viruses , such as B. Herpes of the genital organs ( genital herpes ), herpes of the newborn ( herpes neonatorum ) and encephalitis caused by herpes simplex viruses ( herpes simplex encephalitis ), as well as for infections with varicella-zoster viruses such as shingles ( herpes zoster ).
If the immune system is weakened (congenital or acquired immunodeficiency ), acyclovir is also indicated for the treatment of chickenpox ( varicella ) and infections of the skin and mucous membranes caused by herpes simplex viruses. To prevent herpes simplex infections during immunosuppressive therapy after organ transplants or during radiation therapy , acyclovir is also indicated. In Germany, high doses of acyclovir are also approved for prophylaxis against cytomegalovirus after organ transplants. Certain studies suggest that acyclovir may be effective for this use. However, it has not been established for the general treatment of CMV and is also not the first-line therapy option for prophylaxis after organ transplants. In standard medical works, the active ingredient ganciclovir is usually recommended for this.
Acyclovir is taken as a tablet or suspension or, especially if the disease is severe, administered intravenously. It is important to start therapy as early as possible in the course of the disease and to give it regularly, which does not exceed a time interval of 6 hours.
Externally, acyclovir is used in creams for cold sores ( herpes labialis ) and genital herpes, in eye ointments for herpes simplex infections of the cornea .
Pharmacological properties
Mode of action
Aciclovir is a so-called antimetabolite and in its active (phosphorylated) form inhibits the metabolism of the cell. The special thing about acyclovir is that it is only activated in infected cells. So it only works where it is needed to prevent the virus from replicating. In order to multiply, herpes viruses bring a number of their own enzymes into the cell. These enzymes include B. the viral thymidine kinase . The actual task of thymidine kinase in virus replication is to attach a phosphate group to the natural, cellular thymidine . The thymidine activated in this way is then used by the DNA polymerase of the infected cell to build up the virus DNA , among other things . This is where acyclovir comes in: the viral thymidine kinase of the Alphaherpesvirinae recognizes acyclovir as thymidine and activates it, although the activated form of acyclovir is useless for DNA synthesis . This leads to the chain breaking and virus replication is stopped.
Acyclovir is only converted to the monophosphate form by the viral thymidine kinase. The viral thymidine kinase is far (3000 times) more efficient in phosphorylation than the cellular thymidine kinase. The monophosphate form is then further phosphorylated by the cellular kinase into the active triphosphate form, acyclo-GTP. If Acyclo-GTP is used instead of GTP by the DNA polymerase of the infected cell for DNA replication , this inevitably leads to the termination of the DNA synthesis, since with Acyclo-GTP there is no 3'-OH group to which a the following deoxynucleoside triphosphate (dNTP) could be linked. Acyclo-GTP has approximately 100 times more affinity for viral DNA polymerase than for cellular DNA polymerase. The monophosphate form of acyclovir is also incorporated into the viral DNA , which leads to chain termination during DNA synthesis. It has been shown that viral enzymes cannot remove Acyclo-GMP from the chain, which leads to lasting inhibition of DNA polymerase. Acyclo-GTP is metabolized fairly quickly in the cell, possibly by cellular phosphatases .
The Epstein-Barr virus does not produce the same viral thymidine kinase as the herpes simplex virus and varicella zoster virus . For this reason, cells infected with Epstein-Barr virus cannot convert acyclovir into its pharmacologically active triphosphate form. Aciclovir is therefore ineffective for this member of the herpes virus family.
Pharmacokinetics
The use of acyclovir is limited in part by its low water solubility and low absorption when administered orally, which leads to a bioavailability of less than 50%. In the case of oral administration, the peak concentration in serum is reached after 1–2 hours. Large doses must therefore be administered intravenously. Acyclovir is largely in free form in the blood; only 30% are bound to plasma proteins. The plasma half-life of acyclovir is approximately 3 hours.
Aciclovir is excreted via the kidneys , partly by glomerular filtration , partly by tubular secretion . Kidney problems have become known with large, rapid and intravenous doses because acyclovir can then crystallize in the kidneys .
Side effects
Since acyclovir can also be incorporated into cellular DNA, it is a chromosomal mutagen . It should therefore not be used during pregnancy . In spite of this, neither a teratogenic nor a carcinogenic effect has so far been proven. The acute toxicity ( LD 50 ) of acyclovir when administered orally is above 1 g / kg because of its low absorption in the gastrointestinal tract . In individual cases, extremely high (up to 80 mg / kg) intravenous doses administered by mistake have not shown any side effects. The most common side effects are headache, dizziness and digestive tract discomfort after oral and intravenous administration, as well as stinging and burning sensations when used externally. Resistance to acyclovir develops very quickly , but this hardly limits its clinical use. In a US study from 2002, for example, a resistance rate of 0.2% for cold sore viruses was found, although acyclovir has been used more and more often for the last 20 years.
High levels of CMMG (9-carboxymethoxymethylguanine), the metabolite of acyclovir, have been linked to Cotard's syndrome . This is a clinical picture in which the person concerned is mistakenly convinced that they are dead, that they do not exist, that they believe they have decayed or that they have lost their blood and internal organs. In patients with impaired renal function, this risk appears to persist even after the dose is reduced. In the case cited, dialysis cured Cotard's syndrome within a few hours.
history
Aciclovir was developed in 1974 by Howard Schaeffer and Gertrude B. Elion at Burroughs Wellcome & Company on the basis of nucleosides from a Caribbean sponge (Cryptothetia crypta) discovered in a screening process . Wellcome's search for agents against components of RNA and DNA viruses began in the early 1960s, with a particular search for inhibitors of adenosine deaminase . Clinical testing began in 1977, a patent was granted in 1979 (with Schaeffer as the registered inventor), and a first version was shipped in 1982. Publications on this by the developers appeared from 1977.
Structural variant
Esterification with the amino acid L - valine creates the more absorbable valaciclovir , which acts as a prodrug .
Sales accrual
Without a doctor's prescription, acyclovir is only available in pharmacies in Germany and Austria as a five percent cream or ointment in pack sizes of up to 2 g exclusively for the treatment of cold sores. In Switzerland these preparations for the treatment of cold sores are in dispensing category D , i. In other words, they can be obtained from pharmacies and drug stores after specialist advice.
Trade names
Accarix (A), ACERPES (D), Acic (D), Aciclostad (D), Acivir (CH), Acyclovir (CH), Aviral (CH), Avirox (Malaysia), DYNEXAN herpes cream (D), Helvevir (CH) , HerpoMed (A), Nycovir (A), Supraviran (D), ViroMed (A), Virucalm (CH), Virupos (D), Virzin (D), Xorox (A), Zoliparin (D), Zovirax (D, A, CH) as tablets, suspensions, concentrate for infusion solution, eye ointments and 5% cream; numerous generics (D, A, CH)
Web links
- Acyclovir - a new antiviral drug. Chemotherapy Journal, 1983, accessed May 16, 2014 (with additions Aug 2008).
Individual evidence
- ^ A b F. von Bruchhausen, S. Ebel, AW Frahm, E. Hackenthal: Hagers Handbuch der Pharmazeutischen Praxis. Volume 7: Fabrics A – D. 5th edition. Birkhäuser / Springer, 1991, ISBN 3-540-52688-9 , p. 44.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 698.
- ↑ a b c European Pharmacopoeia 6.2
- ↑ a b c d Pharmacopoeia commentary. Complete works including 36th update delivery 2010, ISBN 978-3-8047-2461-7 .
- ↑ a b Data sheet Acycloguanosine, ≥99% (HPLC), powder from Sigma-Aldrich , accessed on December 21, 2019 ( PDF ).
- ↑ a b c d e f g h i j k l m n o A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag, Stuttgart 2000, ISBN 1-58890-031-2 .
- ^ Patent GB 1 523 865 (Wellcome, August 26, 1977).
- ↑ EM Hodson, M. Ladhani, AC Webster, GFM Strippoli, J. Craig: Antiviral drugs used as protective and preventive therapy reduce CMV disease and CMV-associated deaths in solid organ transplant recipients . In: Cochrane Database of Systematic Reviews. February 2013, p. CD003774.
- ↑ DS Owers, AC Webster, GFM Strippoli, K. Kable, EM Hodson: Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients . In: Cochrane Database of Systematic Reviews. February 2013, p. CD005133.
- ^ BN Fields: Fields Virology. 5th edition. Volume 2, 2007, p. 2750.
- ↑ S. Leflore, PL Anderson, CV Fletcher: A risk-benefit evaluation of aciclovir for the treatment and prophylaxis of herpes simplex virus infections. In: Drug Safety . Volume 23, Number 2, August 2000, pp. 131-142. PMID 10945375 .
- ↑ a b A. J. Wagstaff, D. Faulds, KL Goa: Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. In: Drugs . Volume 47, Number 1, January 1994, pp. 153-205. PMID 7510619 .
- ↑ JC Pottage, HA Kessler: Herpes simplex virus resistance to acyclovir: clinical relevance. In: Infectious agents and disease. Volume 4, Number 3, September 1995, pp. 115-124. PMID 8548189 .
- ^ A b G. D. Morse, MJ Shelton, AM O'Donnell: Comparative pharmacokinetics of antiviral nucleoside analogues. In: Clinical Pharmacokinetics . Volume 24, Number 2, February 1993, pp. 101-123, doi: 10.2165 / 00003088-199324020-00002 . PMID 8453821 .
- ^ A. Schwarz, A. Perez-Canto: Nephrotoxicity of anti-infective drugs. In: International Journal of Clinical Pharmacology and Therapeutics . Volume 36, Number 3, March 1998, pp. 164-167. PMID 9562233 .
- ↑ Safety profile (topical application) (PDF) Assessment report of September 24, 2010 (English).
- ↑ Safety profile (systemic use) (PDF) Assessment report of September 24, 2010 (English).
- ↑ Surveillance for antiviral-agent-resistant herpes simplex virus in the general population with recurrent herpes labialis . GlaxoSmithKline Consumer Healthcare. PMID 12183267 ; Study on resistance to acyclovir.
- ↑ Current management and recommendations for access to antiviral therapy of herpes labialis. In: J Clin Virol. 53 (1), January 2012, pp. 6-11, PMC 3423903 (free full text); Work on the treatment of cold sores (English).
- ↑ Anders Helldén, Ingegerd Odar-Cederlöf, Kajsa Larsson, Ingela Fehrman-Ekholm, Thomas Lindén: Death delusion . In: BMJ . tape 335 , no. 7633 , December 2007, p. 1305-1305 , doi : 10.1136 / bmj.39408.393137.BE , PMID 18156240 .
- ↑ Dannie H. King: History, pharmacokinetics, and pharmacology of acyclovir. In: Journal of the American Academy of Dermatolology. Volume 18, 1988, pp. 176-179. PMID 2828440 .
- ↑ G. Elion, TA Furman, JA Fyfe, P. de Miranda, L. Beauchamp, HJ Schaeffer: Selectivity of action of an anti-herpetic agent, 9- (2-hydroxyethoxymethyl) guanine. In: Proc. Nat. Acad. Sci. Volume 74, 1977, pp. 5716-5720.
- ↑ HJ Schaeffer, L. Beauchamp, P. de Miranda, G. Elion, DJ Bauer, P. Collins: 9- (2-Hydroxyethoxymethyl) guanine activity against viruses of the Herpes group. In: Nature. Volume 272, 1978, pp. 583-585.
- ↑ HJ Schaeffer acyclovir chemistry and spectrum of activity. In: Am. J. Med. 73, 1982, pp. 4-6.
- ↑ G. Elion: Mechanism of action and selectivity of Acyclovir. In: Am. J. Med. 73, 1982, pp. 7-13.
