DNA polymerases
| DNA polymerase | ||
|---|---|---|

|
||
| Ribbon representation of the DNA-binding domain of human DNA polymerase β | ||
| Identifier | ||
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 2.7.7.7 , Transferase | |
| Substrate | Deoxyribonucleoside triphosphate + DNA n | |
| Products | Diphosphate + DNA n + 1 | |
DNA polymerases are enzymes that catalyze the synthesis of DNA from deoxyribonucleotides . DNA polymerases play a key role in DNA replication .
Biochemical aspects
Polymerase activity
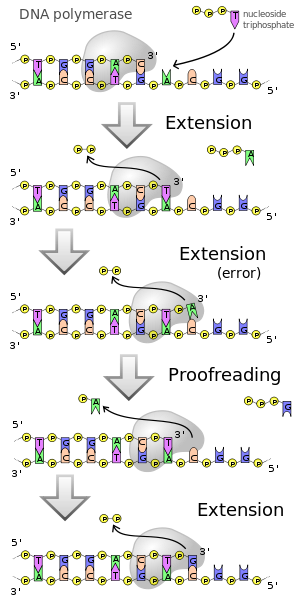
The polymerase enables the chemical linkage of individual molecules ( monomers ) to form a chain ( polymer ). In the case of DNA polymerase, the polymer formed is deoxyribonucleic acid (DNA), deoxyribonucleotides , more precisely deoxy nucleoside triphosphates (dNTPs), serve as monomers . The DNA-dependent DNA polymerase always uses an already existing single strand of DNA as a template (template) for the synthesis of a new, complementary strand, the nucleotide sequence of which is thus determined by the template. This preservation of the DNA sequence is crucial for the ability of DNA polymerase to copy the genetic information encoded in the DNA . Correct copying of the template is achieved through complementary base pairing of the incorporated nucleotide bases with the bases of the DNA template, mediated by hydrogen bonds . The synthesis of the new DNA strand takes place from the 5 'to the 3' end . From a chemical point of view, a nucleophilic attack of the terminal 3'- hydroxyl group of the DNA strand on the α- phosphate of the dNTP takes place, with pyrophosphate being released. This step is catalyzed by the polymerase.
In contrast to RNA polymerases , the synthesis of the complementary DNA strand in DNA polymerases can only take place if the polymerase has a free 3'-hydroxy end available. The first nucleotide is then attached to this. In the polymerase chain reaction (PCR), a single strand of DNA approx. 15-20 nucleotides long ( primer ) is used, which serves as the starting point for the reaction. DNA polymerases usually require magnesium ions as a cofactor .
The catalysis of the formation of the diester bond is functionally analogous to the corresponding reaction of the RNA polymerases. The last nucleotide of the section already synthesized and the nucleotide to be added are each coordinated to one of two magnesium ions in the catalytic center of the polymerase domain. The first phosphate group of the nucleotide to be added is coordinated to both magnesium ions. The spatial position enables the hydroxyl group of the previous nucleotide to attack the phosphate group of the one to be added. A pyrophosphate residue is split off.
Exonuclease activity
Many polymerases also have other enzyme functions. In the presence of low concentrations of dNTPs, the 3 '→ 5' exonuclease activity for removing nucleotides predominates . Some polymerases also have 5 '→ 3' exonuclease activity . To ensure that there are no errors in reading the DNA template, they have this proofreading function (Engl. Proofreading ), ie it can be seen an inappropriate nucleotide able to mount and this is subsequently by to remove the exonuclease activity from the DNA again. This enables an existing DNA or RNA strand that is already paired with the template strand to be broken down while a new strand is being formed. The result is an exchange of the old line for a new line. This exonuclease activity is used in the nick translation method.
Various DNA polymerases
There are three different DNA-dependent DNA polymerases in bacteria such as Escherichia coli . One of them, DNA polymerase I (Pol I), was isolated by Arthur Kornberg in 1955 and was the first polymerase ever to be discovered. However, this is not the most important polymerase in E. coli for replication , since it only catalyzes around 20 synthesis steps (ie it has only a low level of processivity ). However, due to its 5 '→ 3' exonuclease activity, it is responsible for primer degradation during replication. DNA polymerase II and DNA polymerase III , the other two DNA polymerases in E. coli , were not isolated until 15 years after the discovery of DNA polymerase I, after E. coli mutants with a defect in the polymerase I gene turned out to be Proven to be able to replicate . However, these mutants were particularly susceptible to UV radiation and alkylating substances, which is why it is assumed that DNA polymerase I mainly takes on repair tasks. The polymerase III, which carries out the actual replication in E. coli , is made up of a total of seven subunits and only occurs in very few copies per bacterial cell.
Eukaryotic DNA polymerases are classified into the following families:
- Family A: DNA polymerases γ, θ and ν
- Family B: DNA polymerases α, δ, ε and ζ
- Family X: DNA polymerases β, λ, σ and μ
- Family Y: DNA polymerases η, ι and κ
The polymerase γ occurs only in mitochondria .
There are only five DNA polymerases in mammals: α, β, γ, δ, and ε. It is assumed that the polymerases δ and ε, which are decisive for replication, are characterized by high processivity and proof reading . The polymerases α and β, on the other hand, show only low processivity and no proofreading function.
There are also RNA-dependent DNA polymerases that use the RNA as a template and attach dNTPs to it. These are called reverse transcriptase , which also includes telomerase . As independent DNA polymerase as the sole which is terminal deoxyribonucleotidyl known.
Temperature-stable DNA polymerases exist in archaebacteria , which are also used for PCR .
Biological importance
DNA polymerases are central to DNA replication . They enable the faithful copying of genetic information in the form of DNA, thus a decisive step in the reproduction and reproduction of living beings. DNA polymerases also play an important role in the processes involved in the repair of DNA.
Biotechnological importance
In the laboratory, DNA polymerases are often used for the polymerase chain reaction and related methods (e.g. RT-PCR , qPCR ), for nick translation , for random priming and for DNA sequencing . A multitude of different thermostable DNA polymerases , some of which have been modified by protein engineering , are used (e.g. Taq polymerase ). In addition to high temperature stability, thermostable DNA polymerases of archaic origin such as Pfu polymerase also have a proof-reading function , since the DNA produced should not be changed during the PCR. Furthermore, strand-dislocating DNA polymerases such as the φ29 DNA polymerase are used in various methods of isothermal DNA amplification at room temperature . The precursor of the DNA polymerases used today was T4 DNA polymerase .
literature
- Lehninger; David Nelson, Michael Cox: Lehninger Biochemistry. 3rd edition, Springer-Verlag, Berlin Heidelberg 2001, ISBN 3-540-41813-X
- Wilhelm Seyffert: Textbook of Genetics. 2nd edition, Spektrum Akademischer Verlag Heidelberg, Berlin 2003, ISBN 3-8274-1022-3
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry 6th edition, Springer-Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5
Web links
- PDB Molecule of the Month: DNA Polymerase