Diphosphates

Diphosphate (including pyrophosphates , abbreviations PP a and eng. PP i ) are salts and esters of pyrophosphoric acid H 4 P 2 O 7 . Diphosphates are condensates of two phosphates . They are linked to each other via a P – O – P acid anhydride bond ( constitutional formula [(O 3 P) –O– (PO 3 )] 4− ). The esters of these compounds also have a C – O – P bond and have the general constitutional formula R – O - [(PO 2 ) –O– (PO 3 )] 3− (R: organic residue).
Salts of diphosphoric acid
| Salts of diphosphoric acid | ||
|---|---|---|
| Surname | formula | other name |
| Disodium dihydrogen diphosphate | Na 2 H 2 P 2 O 7 | E 450a |
| Trisodium hydrogen diphosphate | Na 3 HP 2 O 7 | E 450b |
| Tetrasodium diphosphate | Na 4 P 2 O 7 | E 450c |
| For more examples see category: Phosphate | ||
synthesis
If secondary phosphates are annealed, diphosphate is formed with elimination of water:
Food chemistry
Diphosphates are used in food chemistry as emulsifiers , among other things , but have a number of other properties and can also function as preservatives , antioxidants , separating and baking agents , complexing agents , acid regulators and melting salts . This artificially produced class of emulsifiers binds water, prevents powdered foods from clumping and, in combination with calcium, leads to a creamy consistency. Since phosphates are suspected of triggering hyperactivity , allergic reactions and osteoporosis , the correct dosage should always be observed when taking phosphates. A permissible daily dose of 70 milligrams per kilogram of body weight was set for the total amount of phosphoric acid and phosphates ingested . In the EU , diphosphates ( disodium , tri-sodium, tetrasodium , tetrapotassium, dicalcium and calcium dihydrogen diphosphate) are permitted as food additives with the number E 450 for certain foods, each with different maximum quantity restrictions. According to the Additive Admissions Ordinance , these are - largely uniform for most of the approved phosphates - individual specifications for a wide range of numerous different types of food. The maximum permitted amounts vary from 0.5 to 50 grams per kilogram (in creamer for vending machines) or the lack of a fixed limit ( quantum satis - as required, for food supplements and sometimes for chewing gum).
Diphosphoric acid esters
Diphosphoric acid esters play a significant role in biochemistry (see below). As anthropogenic xenobiotics , they play a rather minor role.
The tetraethyl ester of the diphosphoric acid tetraethyl pyrophosphate (TEPP) has achieved a certain importance as a human-toxic nerve poison and an unapproved insecticide .
Biochemical significance
Like all phosphoric acid, diphosphates can exergonically transfer anhydride phosphate groups to nucleophilic molecules such as water, alcohols and other compounds with OH groups . Such phosphorylation reactions play a crucial role in all biochemical processes in which energy is transferred. ATP plays a decisive role in this .
Like polyphosphate, diphosphate can arise spontaneously in geochemical processes . When complex organic molecules emerged during chemical evolution in the late Hadean , diphosphate could have played the role of the energy carrier that ATP plays today.
In the human metabolism, salts of diphosphoric acid (“inorganic pyrophosphate”) are more like waste products that have to be recycled. Calcium pyrophosphate crystals are involved in pseudogout (crystal arthropathy ) in a painful disease of the joints.
Organic diphosphate derivatives ("organic pyrophosphate"), on the other hand, are indispensable for all known living beings.
Organic diphosphates
Esters of pyrophosphoric acid is found in all living organisms. They are the basic building blocks of a large number of complex natural substances. The high- energy pyrophosphate groups provide some of the energy required for their biosynthesis . Dimethylallyl pyrophosphate (dimethyl-allyl-PP), together with isopentenyl pyrophosphate, is the starting material for cholesterol biosynthesis and the biosynthesis of 30,000 known terpenes and terpenoids .
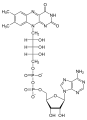
- Diphosphates as coenzymes
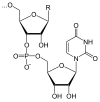
A number of coenzymes (see figure on the right), which are vital for all organisms and which are consistently produced in the metabolism, belong to the organic diphosphates. (In humans, the diphosphate riboflavin, vitamin B 2, must be taken in with food.)
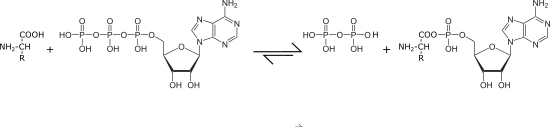
With the coenzyme ADP ( adenosine diphosphate ), the diphosphate group plays an essential role in energy transfer processes in living cells. ADP is a co-substrate of almost all enzymes with which ATP is regenerated using energy ( ΔH = 50 kJ / mol under physiological conditions). This happens mainly by means of the ATP synthase according to the reaction equation
- ADP + phosphate + H + outside → ATP + H 2 O + H + inside .
according to the principle of chemiosmosis . In the substrate- ADP is also such reactants that. B. in phosphoglycerate kinase , the following reaction catalyzes:
The reverse reaction of this equation, which is important in the biosynthesis of sugars , reverses the formation of ADP. A whole series of endergonic reactions occur in the metabolism according to this principle
- ATP + H 2 O → ADP + phosphate
where a group transfer potential ΔG 0 '= −30.5 [kJ · mol −1 ] supplies the energy.
Inorganic diphosphates
Diphosphate as a waste product of anabolic processes
ATP is also consumed in processes in which it is not organic adenosine diphosphate but inorganic diphosphate (abbreviated: PPi ) that is formed. In summary, this happens after
- ATP + H 2 O → AMP + PPi (diphosphate)
with a group transfer potential ΔG 0 '= −57 [kJ · mol −1 ]
In the metabolism, diphosphate is always formed in anabolic reactions that are used to build up biomass. Of approx. 200 PPi producing reactions, approx.
- 7% of the production of molecules that regulate the metabolism, such as B. the adenylyl cyclases catalyzed formation of cyclo-AMP .
- Around 20% result from the synthesis of fatty acids, but also their breakdown ( α-oxidation and β-oxidation ).
- 29% of the resulting PPi is released from small, mostly essential biomolecules during biosynthesis.
- This also includes the formation of "activated sulfuric acid" in the first step of desulfurization and sulfur assimilation in plants .
- Also the synthesis of NAD , which after
- ATP + NMN ⇌ NAD + PPi
- catalyzed by nicotinamide nucleotide adenylyl transferase , releases diphosphate. In principle, the reverse of this reaction (NAD + PPi ⇌ ATP + NMN) can also use diphosphate to form ATP. But under physiological conditions the cleavage of such an important coenzyme as NAD cannot be a relevant reaction for the formation of ATP. In fact, NAD is hydrolytically degraded in vivo by a nucleotide diphosphatase (see also Organic_Diphosphatases ), and the reverse reaction of the adenylyl transferase must be avoided.
- Approx. 35% of the diphosphate comes from the synthesis and modification of macromolecules. In principle, all syntheses of biological macromolecules release diphosphate.
- Biosynthesis of nucleic acids, e.g. B. by means of RNA polymerase II , which also releases PPi from other nucleotide triphosphates ( transcription ):
 + +
+ +

- The DNA - replication uses inorganic diphosphate free at every step.
- The formation of "activated amino acids" by the respective aminoacyl-tRNA synthetases as the first step in protein biosynthesis provides the most PPi in most organisms:
- Biosynthesis of nucleic acids, e.g. B. by means of RNA polymerase II , which also releases PPi from other nucleotide triphosphates ( transcription ):
The splitting off of the diphosphate releases energy because the resulting free diphosphate is stabilized by mesomerism and hydration on the one hand. On the other hand, there is also an entropic effect, since the entropy of the system has increased. This energy is often used to enable an endergonic reaction that is coupled to this process . Diphosphates are often split into two phosphates by pyrophosphatases . This shifts the respective reaction equilibria even more to the right, which makes the respective reactions irreversible, because the pyrophosphatase removes an end product from the equilibrium.
Use of diphosphate in metabolism
It is also discussed whether diphosphates can be used as alternative energy donors in bacteria and plants in addition to ATP .
In animal metabolism and in the metabolism of many microorganisms, diphosphate is quickly recycled to phosphate by pyrophosphatases dissolved in the cytosol and the energy released is released as heat.
Apart from multicellular animals and fungi, diphosphate is also used for energy. This happens through insoluble pyrophosphatases integrated in biomembrane , which act as chemiosmotic active proton pumps. These enzymes are very old and go back to a common family tree. Apart from many prokaryotes and animal protozoa, they play an important role in land plants. They are found there in the membranes of vacuoles , where they serve as H + pumps. In doing so, they save ATP, which would otherwise have to be consumed by ATP-splitting H + pumps. In plant mitochondria , diphosphate can be split by the specific diphosphate-splitting activity of the ATP synthase without energy conservation.
In the cell plasma of many plants there are no soluble, energy-wasting pyrophosphatases, and so the diphosphate concentration in the cytosol assumes values of 0.2-0.3 mM. Processes using diphosphate are widespread in plants and occur in parallel to metabolic pathways that use ATP. Pyrophosphate can serve as an energy carrier to replace ATP, for example in glycolysis , where a pyrophosphate: fructose-6-phosphate phosphotransferase replaces the phosphofructokinases .
Individual evidence
- ↑ L. Szinicz: History of chemical and biological warfare agents . In: Toxicology . tape 214 , no. 3 , 2005, p. 173 , doi : 10.1016 / j.tox.2005.06.011 ( online ).
- ↑ a b c Alexander A. Baykov, Anssi M. Malinen, Heidi H. Luoto, Reijo Lahti: Pyrophosphate-Fueled Na + and H + Transport in Prokaryotes . In: Microbiol. Mol. Biol. Rev. Band 77 , no. 2 , 2013, p. 267-276 , doi : 10.1128 / MMBR.00003-13 ( online [PDF]).
- ^ Nils G. Holm, Herrick Baltscheffsky: Links between hydrothermal environments, pyrophosphate, Na +, and early evolution. In: Origins of Life and Evolution of Biospheres . tape 41 , no. 5 , 2011, p. 483-493 , doi : 10.1007 / s11084-011-9235-4 .
- ^ A b Lubert Stryer: Biochemistry . 5th edition. WH Freeman and Company, New York 2002, ISBN 0-7167-1843-X . , quoted from en: Adenosine triphosphate
- ↑ Jukka K. Heinonen: Biological role of inorganic pyrophosphate . Springer Science & Business Media (formerly Kluwer Academic Publ), Berlin 2001.
- ^ Arthur Kornberg: The participation of inorganic pyrophosphate in the reversible enzymatic synthesis of diphosphopyridine nucleotide. In: Journal of Biological Chemistry . tape 176 , no. 3 , 1948, p. 1475-1476 .
- ↑ Jukka K. Heinonen: Biological role of inorganic pyrophosphate . Springer Science & Business Media (formerly Kluwer Academic Publ), Berlin 2001, p. 11 .
- ^ A. Serrano et al .: H + -PPases: yesterday, today and tomorrow . In: IUBMB Life. 59 (2), 2007, pp. 76-83. PMID 17454298 .
- ↑ M. Baltscheffsky: Inorganic pyrophosphate as an energy donor in photosynthetic and respiratory electron transport phosphorylation systems . In: Biochem Biophys Res Commun . 28 (2), 1967, pp. 270-276. PMID 4291991 .
- ↑ Marco Zancani, Valentino Casolo, Carlo Peresson, Giorgio Federici, Andrea Urbani, Francesco Macrı̀, Angelo Vianello: The β-subunit of pea stem mitochondrial ATP synthase exhibits PPiase activity . In: Mitochondrion . tape 3 , no. 2 , 2003, p. 111–118 , doi : 10.1016 / S1567-7249 (03) 00105-3 ( online [PDF]).
- ↑ M. Stitt: Pyrophosphate as an alternative energy donor in the cytosol of plant cells: an enigmatic alternative to ATP . In: Bot. Acta . tape 111 , 1998, pp. 167-175 .
- ↑ Yoko Chiba, Ryoma Kamikawa, Kumiko Nakada-Tsukui, Yumikoco Saito-Nakano, Tomoyoshi Nozaki: Discovery of PPi-type Phosphoenolpyruvate Carboxykinase Genes in Eukaryotes and Bacteria . In: Journal of Biological Chemistry . tape 290 , no. 39 , 2015, p. 23960-23970 , doi : 10.1074 / jbc.M115.672907 ( abstract ).