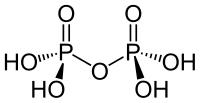
Diphosphoric acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Diphosphoric acid | |||||||||||||||||||||
| other names |
Pyrophosphoric acid |
|||||||||||||||||||||
| Molecular formula | H 4 P 2 O 7 | |||||||||||||||||||||
| Brief description |
white to light yellow solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 177.98 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
61 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
very good in water (7090 g l −1 at 23 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Diphosphoric acid (also called pyrophosphoric acid ) is an oxo acid of phosphorus and belongs to the inorganic acids . It is derived from phosphoric acid and can be represented as an agglomeration of two phosphoric acid molecules with elimination of water .
Diphosphoric acid is highly hygroscopic and a moderately strong inorganic acid . Diphosphoric acid hydrolyzes exothermically to phosphoric acid with water .
The salts and esters of diphosphoric acid are called diphosphates or pyrophosphates .
Extraction and presentation
Diphosphoric acid is produced according to the above formula by dehydrating phosphoric acid at 200-300 ° C. Pure diphosphoric acid is made from phosphoric acid and phosphorus oxychloride :
See also
Individual evidence
- ↑ a b c sheet diphosphoric from Acros, accessed on 26 February 2010 .
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ a b Pyrophosphoric acid data sheet from Sigma-Aldrich , accessed on March 28, 2011 ( PDF ).
- ↑ Entry on diphosphoric acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ↑ Georg Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. 2nd edition. Volume 1. Academic Press, New York et al. 1963, pp. 546-547.



