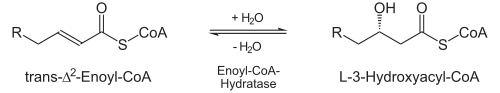β-oxidation
| Parent |
| Fatty acid oxidation |
| Subordinate |
| via acyl-CoA dehydrogenase via acyl-CoA oxidase |
| Gene Ontology |
|---|
| QuickGO |

The biochemical breakdown mechanism of fatty acids is called β-oxidation . The term refers to the held on β-carbon atom of the fatty acid oxidation . The β-oxidation was formerly also known as the fatty acid spiral.
The β-oxidation was discovered in 1904 by Franz Knoop in Freiburg. The exact mechanism of this metabolic pathway was not clarified until 50 years later. In animal cells, β-oxidation takes place largely in the mitochondria , in plant cells in the glyoxysomes .
Proteins of the following genes are involved in β-oxidation: CPT1A , CPT1B , CPT1C , CPT2 , HSD17B4 , ECH1 , HADHA , HADHB , ECHS1 , EHHADH , ECI1 , HADH , CROT .
preparation
Before the actual β-oxidation can begin, the otherwise very inert fatty acids must first be "activated" in the cytosol and then transported from the cytosol into the matrix of the mitochondria, where the β-oxidation takes place.
Activation of the fatty acid
The aim of activation is the formation of acyl-CoA by transferring the fatty acid to coenzyme A. This creates an energy-rich thioester bond , which enables the further reaction steps. In the first step, ATP is split into pyrophosphate and AMP , which is used directly to form acyl-AMP (also: acyl-adenylate). Parallel to the cleavage of the pyrophosphate into simple phosphate by a pyrophosphatase, the fatty acid can be esterified with coenzyme A with the cleavage of the AMP due to the energy released. The activated form of the fatty acid is called acyl-CoA. Both reactions are catalyzed by a fatty acid CoA ligase .
Transport into the mitochondrial matrix
The acyl group is then transferred to carnitine with the cleavage of coenzyme A by the enzyme carnitine acyltransferase I and actively transported into the matrix of the mitochondria. This process is catalyzed by the carnitine-acylcarnitine transporter (CACT), which in the antiport transports acyl-carnitine into the mitochondrial matrix and at the same time transports carnitine out. In the matrix, the acyl residue is detached from carnitine by carnitine acyl transferase II and transferred back to coenzyme A. While the activated fatty acid is now available for degradation, the carnitine is exported back into the cytosol by the CACT. The acyl-CoA activation is not reversible: an activated fatty acid is broken down.
Actual β-oxidation
Depending on the type of fatty acid (number of carbon atoms, position and configuration of any double bonds), the process of degradation can differ from that of the even-numbered, saturated fatty acids, since additional reactions may be necessary in order to find suitable substrates for the enzymes of β-oxidation or because reaction products other than acetyl-CoA are produced.
Breakdown of even-numbered, saturated fatty acids
The actual degradation can be divided into four successive steps:
FAD-dependent oxidation
- On the acyl-CoA, the enzyme acyl-CoA dehydrogenase creates a trans double bond between carbon atoms 2 (C α ) and 3 (C β ) . This is unusual for unsaturated fatty acids, which are usually in the cis configuration, but necessary because the enzyme in the next step, the enoyl-CoA hydratase, only recognizes fatty acids in the trans configuration. This process also reduces one FAD to FADH 2 .
Hydration
- Enoyl-CoA hydratase stereospecifically adds water to the newly formed double bond, namely to the β-carbon atom. This creates L-3-Hydroxyacyl-CoA (also: L-β-Hydroxyacyl-CoA).
NAD + dependent oxidation
- In the next reaction, the C 3 -hydroxy group is oxidized to a keto group by L -3-hydroxyacyl-CoA dehydrogenase (also: β-hydroxyacyl-CoA dehydrogenase). The cofactor here is NAD + , which absorbs the electrons that are created and is thus reduced to NADH + H + . This step is the eponymous for the entire mechanism. One example is the dehydrogenation of 2-hydroxystearate to 2-oxostearate by 2-hydroxy fatty acid dehydrogenase .
Thiolysis
- When a coenzyme A is absorbed, the enzyme 3-keto-thiolase splits off acetyl-CoA (activated acetic acid ), and a fatty acid molecule shortened by two carbon atoms (in the form of acyl-CoA ) remains, which can be returned to the first step.
- This sequence of reactions is repeated until two acetyl-CoA are left at the end.
Breakdown of odd-numbered fatty acids
The breakdown of these fatty acids differs from that of the even-numbered ones in that in the end it is not acetyl-CoA but propionyl-CoA that remains. This is now converted in several steps to succinyl-CoA , a metabolite of the citrate cycle .
For this purpose, the propionyl-CoA is first carboxylated on the α-carbon atom with cleavage of an ATP . This reaction is catalyzed by propionyl-CoA carboxylase , which contains biotin (vitamin B 7 ) as a cofactor . The result ( S ) -methylmalonyl-CoA , which by the methylmalonyl-CoA racemase in (in the next step R is converted) -methylmalonyl-CoA. Finally, the carboxy group is transferred by the methylmalonyl-CoA mutase , vitamin B 12 -dependent, from the α-C atom to the carbon atom of the methyl group, whereby succinyl-CoA is formed, which can be added to the citrate cycle.
Breakdown of unsaturated fatty acids
Since most of the double bonds of naturally occurring unsaturated fatty acids have a cis configuration, but the β-oxidation enzymes only accept substrates in the trans configuration, these must first be converted by specific isomerases. Another problem is posed by double bonds that follow one another directly (-CH = CH-CH = CH-). These must be reduced in such a way that only one double bond (-CH 2 -CH = CH-CH 2 -) remains in order to get from to be recognized by the enzymes.
Energy yield
The acetyl-CoA formed during the β-oxidation can either be broken down further in the citrate cycle or used for the synthesis of keto bodies . In the case of degradation, one FADH 2 and one NADH + H + are produced per round of β-oxidation , which deliver 1.5 and 2.5 ATP respectively via the respiratory chain. Every acetyl-CoA that is broken down via the citrate cycle also enables the synthesis of 10 ATP. For example, when one molecule of palmitic acid is completely broken down, 106 molecules of ATP can be formed: Palmitic acid contains 16 carbon atoms and is therefore broken down into a total of eight acetyl-CoA, with seven molecules each of FADH 2 and NADH + H + being formed, as the cycle goes through seven times becomes. However, since to activate the fatty acid in the cytosol, an ATP was split by hydrolysis of two energy-rich compounds to form AMP, the net result is: 7 × 4 + 8 × 10 - 2 = 106 ATP. In comparison, if one molecule of glucose is broken down completely, only 32 molecules of ATP are produced.
β-oxidation in other organelles
Fatty acids are not only broken down in the mitochondria. In plants and yeasts, for example, fatty acids are broken down exclusively in the glyoxysomes or peroxisomes . In humans, very long-chain fatty acids (at least 22 carbon atoms) are first broken down into shorter-chain products in the peroxisomes. Longer-chain, rare fatty acids (26 to 28 carbon atoms with several double bonds) are also metabolized by peroxisomes in brain cells. These truncated fatty acids can then be metabolized by mitochondrial β-oxidation as described above.
The ALD protein is used instead of carnitine to transport long-chain fatty acids into the human peroxisome. If this has a defect, this leads to the development of a disease, the X-adrenoleukodystrophy .
The breakdown of fatty acids in peroxisomes has certain peculiarities: The first enzyme oxidizes the fatty acid activated by coenzyme A directly using oxygen. This creates a trans -Δ 2 -enoyl-CoA and hydrogen peroxide (H 2 O 2 ). This reaction is catalyzed by an acyl-CoA oxidase ( EC 1.3.3.6 ) and bypasses the transfer of electrons to FAD (see above). H 2 O 2 is disproportionated to oxygen and water by a catalase . In addition, the activities of the two following enzymes (enoyl-CoA hydratase; L -3-hydroxyacyl-CoA dehydrogenase) are combined in a multifunctional enzyme. After all, peroxisomal thiolase does not split fatty acids whose chain length is shorter than eight carbon atoms.
Reverse β-oxidation
The reversal of β-oxidation does not take place in nature, although there is no fundamental obstacle. This reversal would even be more efficient than normal fatty acid synthesis and, if implemented in the appropriate microorganisms, could efficiently produce biofuels and raw materials. Rice University in Houston succeeded in doing this in the model organism E. coli in 2011 and is an example of successful bioengineering . For this purpose, 1. partial routes for shorter and longer chains had to be deregulated and put together; 2. the competing glucose fermentation is switched off; 3. terminating enzymes for the desired products (acyl-CoA reductase, aldehyde / alcohol dehydrogenase, thioesterase) are inserted / overexpressed and 4. initiating enzymes (thiolases) are added for the desired starting materials.
Individual evidence
- ↑ uniprot.org
- ^ A b Donald Voet, Judith G. Voet: Biochemistry. 3. Edition. Wiley & Sons, 2004, ISBN 0-471-19350-X , p. 927.
- ↑ Geoffrey Zubay: biochemistry. 4th edition. Mcgraw-Hill Professional, 1999, ISBN 3-89028-701-8 , p. 488.
- ^ S. Kemp, RJ Wanders: X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment. In: Mol Genet Metab. 90 (3) 2007, pp. 268-276. PMID 17092750 , doi: 10.1016 / j.ymgme.2006.10.001
- ^ HW Moser et al.: X-linked adrenoleukodystrophy. In: Nat Clin Pract Neurol. 3 (3) 2007, pp. 140-151. PMID 17342190 , doi: 10.1038 / ncpneuro0421
- ↑ C. Dellomonaco, JM Clomburg et al: Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. In: Nature . Volume 476, Number 7360, August 2011, pp. 355-359. doi: 10.1038 / nature10333 . PMID 21832992 .
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Biochemistry. 6th edition. Spectrum Akademischer Verlag, Heidelberg 2007, ISBN 978-3-8274-1800-5 .
- H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn, Carsten Biele (translator): Biochemie. 4th updated edition. Pearson Studium, 2008, ISBN 978-3-8273-7312-0 , pp. 667ff.
- Joachim Rassow , Karin heim, Roland Netzker, Rainer Deutzmann: Dual Series - Biochemistry. 1st edition. Thieme, 2006, ISBN 3-13-125351-7 .
See also
Web links
- Pedro Silva: The chemical logic behind fatty acid metabolism. (engl.)