Nicotinamide adenine dinucleotide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
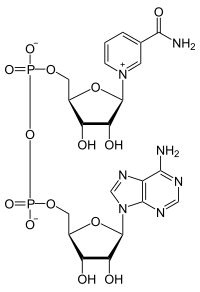
|
||||||||||||||||||||||
| NAD + (oxidized form) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Nicotinamide adenine dinucleotide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
colorless, hygroscopic powder ( oxidized form, inner salt ) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass |
|
|||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
140–142 ° C (decomposition) ( oxidized form, inner salt ) |
|||||||||||||||||||||
| solubility |
little in water (10 g l −1 ) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Nicotinamide adenine dinucleotide ( nicotinamide - adenine - dinucleotide , abbreviated NAD ) is a coenzyme that is formally a hydride transfers (two electrons , in short: 2 e - , and a proton , H + ). It is involved in numerous redox reactions of the metabolism of the cell involved.
The IUPAC / IUBMB suggest the abbreviations NAD + for the oxidized form, NADH + H + for the reduced form and NAD in general. Sometimes there is NAD instead of NAD + and NADH 2 instead of NADH + H + . The spelling of NADH 2 is incorrect because the protons bind to different places on the molecule.
The coenzyme was discovered in 1906 by Arthur Harden and William Young (Harden and Young esters). NAD + was in the older literature until the early 1960s, also known as Diphosphopyridinnucleotid abbreviated DPN , or under the name Codehydrase I , Codehydrogenase I or coenzyme I known.
Compared to nicotinamide adenine dinucleotide phosphate (NADP + ) and nicotine acid adenine dinucleotide phosphate (NAADP), two coenzymes otherwise almost identically structured, both at 2 'C atom of adenosine another phosphate have radical, is there at the NAD just a normal hydroxyl .
chemistry
Redox reaction of NAD: NAD + can be reduced to NADH by taking up two electrons (e - ) and one proton (H + ).
biochemistry
function
According to the Nernst equation, the redox potential of the redox couple NAD + / NADH depends on the concentration ratio NAD + / NADH. If this is large, the redox potential is more positive (higher oxidizing capacity); if this is small, it is more negative (higher reducing power). Since NAD + mostly serves as an oxidizing agent in the organism , the NAD + / NADH ratio is large (≫1). In contrast, NAD P H, which has a correspondingly low ratio of NADP + / NADPH (≪1), is primarily used as the reducing agent . The fact that a single redox pair could not simultaneously provide a high redox potential for biological oxidations and a low redox potential for biological reductions is the reason why there are two differentiable redox cofactors .
The high-energy, reduced form NADH serves in the oxidative metabolism as an energy-supplying coenzyme of the respiratory chain , whereby ATP is generated. When it is oxidized, it releases the electrons previously absorbed in the catabolic glucose and / or fat metabolism and transfers them to oxygen. This ultimately results in NAD + and hydrogen.
NAD + is also a coenzyme of dehydrogenases , e.g. B. alcohol dehydrogenase (ADH), which oxidize alcohol.
biosynthesis
NAD + is produced in the body from nicotinic acid (niacin, vitamin B3) and nicotinamide as well as from the breakdown products of the amino acid tryptophan . Since both starting materials are essential, deficiency symptoms such as Pellagra are possible, but rather rare in Europe because of the two possible metabolic pathways .
The hub of both reaction pathways is nicotinate D- ribonucleotide , which can be formed directly from nicotinic acid using nicotinate phosphoribosyl transferase , or which is formed from the tryptophan breakdown product quinolinic acid using the enzyme quinolinate phosphoribosyl transferase . The latter reaction takes place mainly in the liver . In the next step, adenosine phosphate is added to nicotinate D-ribonucleotide. This reaction is catalyzed by nicotinamide nucleotide adenylyl transferase , and deamido-NAD + is formed . This is finally aminated to NAD + by means of NAD synthase .
Another synthetic route begins with nicotinamide, which is converted to the dinucleotide with nicotinamide phosphoribosyl transferase ; this is already an amide , so that only the transfer of adenosine phosphate with the above. Transferase is necessary to obtain NAD + .
The high-energy, reduced form of NADH is produced in catabolism (in glycolysis and in the citric acid cycle ).
Absorption properties
The nicotinamide adenine dinucleotide has an identical adenine region in both its reduced (NADH) and its oxidized (NAD + ) form (cf. structural formula). This absorbs light at a wavelength of 260 nm, which explains the common absorption maximum in the 260 nm range shown in the diagram. It is noticeable here that the absorption of NAD + in this range is higher than that of NADH at the same concentration of substances . The reason for this is that the absorption of the oxidized, mesomeric nicotinamide ring , which also absorbs at 260 nm, is superimposed on the absorption of adenine and thus ensures the increased absorption at 260 nm. If the nicotinamide ring is reduced, a quinoid system is created that absorbs light with a wavelength of 340 nm. The molar extinction coefficient ԑ of NADH (as well as NADPH) at 340 nm is ԑ = 6300 l / (mol · cm).
This difference between NAD + and NADH in the UV spectrum makes it possible to observe the conversion between the oxidized and reduced form of the coenzyme in a spectrophotometer. For example, the oxidation of NADH or the reduction of NAD + can be observed photometrically in an enzyme assay if the enzyme used uses NAD + as substrate. The amount of converted substrate can be monitored photometrically by the change in absorption at 340 nm; the concentration can then be determined with the aid of Lambert-Beer’s law . Since this is proportional to the amount of converted cosubstrate, indirect quantitative statements about the amount of converted substrate and manufactured product are possible. Direct tracking of the substrate and product concentration is often more difficult.
Individual evidence
- ↑ a b c d Entry on nicotinamide adenine dinucleotide. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ↑ a b c Data sheet NAD + , Free Acid - CAS 53-84-9 - Calbiochem (PDF) from Merck , accessed on December 21, 2019.
- ↑ A. Harden, WJ Young: The alcoholic ferment of yeast juice. In: Proceedings of the Royal Society of London (Series B, Containing Papers of a Biological Character ed.) , Volume 78, No. 526, October 1906, pp. 369-375. ( doi : 10.1098 / rspb.1906.0029 ).
- ↑ M. Nakamura, A. Bhatnagar, J. Sadoshima: Overview of pyridine nucleotides review series. In: Circulation research. Volume 111, Number 5, August 2012, pp. 604-610. ( doi : 10.1161 / CIRCRESAHA.111.247924 ; PMID 22904040 ; PMC 3523884 (free full text)).
- ↑ C. Cantó, J. Auwerx: NAD + as a signaling molecule modulating metabolism. In: Cold Spring Harbor symposia on quantitative biology. Volume 76, 2011, pp. 291-298 ( doi : 10.1101 / sqb.2012.76.010439 ; PMID 22345172 ; PMC 3616234 (free full text)).
- ↑ Fukuwatari T, Morikawa Y, Hayakawa F, Sugimoto E, Shibata K: Influence of adenine-induced renal failure on tryptophan-niacin metabolism in rats . In: Bioscience, Biotechnology, and Biochemistry . 65, No. 10, October 2001, pp. 2154-2161. PMID 11758903 .
- ↑ HU Bergmeyer: New values for the molar extinction coefficients of NADH and NADPH for the use in routine laboratories . In: Journal of Clinical Chemistry and Clinical Biochemistry . tape 13 , no. 11 . Walter de Gruyter, Berlin November 1975, p. 507-508 , PMID 3038 .




