Nicotinamide
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
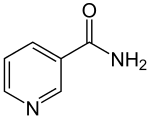
|
|||||||||
| General | |||||||||
| Common name | Nicotinamide | ||||||||
| other names |
|
||||||||
| Molecular formula | C 6 H 6 N 2 O | ||||||||
| CAS number | 98-92-0 | ||||||||
| PubChem | 936 | ||||||||
| ATC code | |||||||||
| DrugBank | DB02701 | ||||||||
| Brief description | White dust | ||||||||
| Occurrence | Liver, mammalian muscles, yeast, milk, grain seedlings | ||||||||
| physiology | |||||||||
| function | Human anti pellagra -vitamin, component of the coenzymes NADH and NADPH | ||||||||
| Daily need | 2-17 mg | ||||||||
| Consequences in case of deficiency | Loss of appetite, weight loss, decline in physical and mental performance, sleep disorders, upset, confused states, memory disorders, burning tongue, diarrhea, pellagra | ||||||||
| Overdose | Nausea, headache, urticaria, tiredness, facial rigidity and adaptation disorders of the eyes were recorded in patients who took 3 g nicotinamide daily for 3 to 36 months. Liver damage (hepatotoxicity, jaundice and cholestatic hepatitis) was observed after very high doses (up to 9 g daily). | ||||||||
| properties | |||||||||
| Molar mass | 122.12 g mol −1 | ||||||||
| Physical state | firmly | ||||||||
| density | 1.4 g cm −3 (20 ° C) | ||||||||
| Melting point |
|
||||||||
| solubility | easily in water (691 g l −1 at 20 ° C) | ||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Nicotinamide is the amide of nicotinic acid (niacin, vitamin B 3 ). It is of great biochemical importance as an important component of the coenzymes NAD + and NADP + , which are capable of reversible hydrogen bonding. The pyridine ring is reduced, whereby the nitrogen loses its positive charge.
synthesis
Nicotinamide is made on the basis of acrolein and ammonia . These react to β-picoline , which to 3-cyanopyridine (nicotinonitrile) ammonoxidiert is. The hydrolysis of this leads to nicotinamide.
use
Pellagra is a common skin disease in people who eat mainly millet or corn . This disease is due to insufficient intake of nicotinic acid, nicotinamide or their biochemical precursor L - tryptophan . That is why nicotinamide is used as a medicinal substance (Nicobion ® ).
In a phase 3 multicenter study with 380 skin cancer patients who took 1000 mg nicotinamide daily for one year, the active ingredient reduced the risk of new non-melanomatous skin cancer lesions by 23% compared to placebo.
Nicotinamide, given in high doses prior to radiation therapy for cancer patients, could increase the response of certain tumors. There are indications of effectiveness against multiresistant germs such as MRSA and Pseudomonas .
Animal experiments on transgenic mice showed that nicotinamide was able to prevent the animals from losing their memory. Hence the possibility that it may also play a role in the treatment of Alzheimer's patients. In the nematode Caenorhabditis elegans , the addition of nicotinamide to food resulted in a 10% increase in life expectancy, which the participating researchers attributed to the increased binding of free radicals and the associated reduced oxidative stress .
In cosmetic products such as hair or skin care products, nicotinamide is said to have a smoothing effect and neutralize irregularities on the skin surface.
Individual evidence
- ↑ entry to niacinamide in CosIng database of the European Commission, accessed on February 26 2020th
- ↑ a b Data sheet Nicotinamide from AlfaAesar, accessed on April 14, 2010 ( PDF )(JavaScript required) .
- ↑ DGE : The reference values for nutrient intake. DA-CH reference values of the DGE, ÖGE, SGE / SVE: niacin.
- ↑ NICOTINEAEUREAMID 200 mg Jenapharm Tabl. ( Memento from December 18, 2012 in the web archive archive.today ) - Description
- ↑ a b c d Hino, T .; Ford, JL; Powell, ML: Assessment of nicotine amide polymorphs by differential scanning calorimetry in Thermochim. Acta 374 (2001) 85-92.
- ↑ a b c d Entry on nicotinamide in the GESTIS substance database of the IFA , accessed on April 27, 2017(JavaScript required) .
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta , Zurich, 2006, ISBN 3-906390-29-2 , p. 491.
- ^ Siegfried Ebel and Hermann J. Roth (editors): Lexikon der Pharmazie, Thieme Verlag, 1987, ISBN 3-13-672201-9 , p. 463.
- ↑ Andrew C. Chen, Andrew J. Martin, Bonita Choy, Pablo Fernández-Peñas, Robyn A. Dalziell, Catriona A. McKenzie, Richard A. Scolyer, Haryana M. Dhillon, Janette L. Vardy, Anne Kricker, Gayathri St. George, Niranthari Chinniah, Gary M. Halliday, Diona L. Damian: A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. In: New England Journal of Medicine. 373, 2015, pp. 1618–1626, doi: 10.1056 / NEJMoa1506197 , PMID 26488693 .
- ^ NICE study list , accessed August 1, 2016
- ↑ P. Kyme, NH. Thoennissen, CW. Tseng, GB. Thoennissen, AJ. Wolf, K. Shimada, UO. Krug, et al .: C / EBPε mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice . In: J Clin Invest . 122, No. 9, September 2012, pp. 3316-29. doi : 10.1172 / JCI62070 . PMID 22922257 .
- ↑ Vitamin B3: New weapon against MRSA? In: Doctors newspaper. September 20, 2012, accessed May 17, 2013 .
- ↑ KN. Green, JS. Steffan, H. Martinez-Coria, X. Sun, SS. Schreiber, LM. Thompson, FM. LaFerla: Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau . In: J Neurosci . 28, No. 45, November 2008, pp. 11500-11510. doi : 10.1523 / JNEUROSCI.3203-08.2008 . PMID 18987186 .
- ↑ K. Schmeisser, et al .: Role of Sirtuins in Lifespan Regulation is Linked to Methylation of Nicotinamide . In: Nature Chemical Biology . 2013. doi : 10.1038 / nchembio.1352 .
- ↑ Entry of nicotinamide on haut.de , accessed on October 7, 2016.
