Acrolein
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Acrolein | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 4 O | |||||||||||||||
| Brief description |
colorless to yellowish, easily mobile liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 56.06 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.84 g cm −3 |
|||||||||||||||
| Melting point |
−88 ° C |
|||||||||||||||
| boiling point |
52 ° C |
|||||||||||||||
| Vapor pressure |
295 h Pa (20 ° C) |
|||||||||||||||
| solubility |
good in water (267 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4017 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Acrolein [ acrolein ] (also propenal , acrylaldehyde , 2-propenal , or prop-2-enal , or AQUALIN ) is a chemical, and as aldehyde of organic chemistry assigned. It is a clear liquid substance that has the molecular formula C 3 H 4 O.
Extraction and presentation
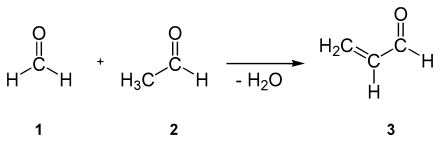
Acrolein ( 3 ) can be obtained by partial oxidation of propene or by reaction of acetaldehyde ( 2 ) with formaldehyde ( 1 ) ( aldol condensation ):
This method has now been replaced by the Sohio process .
Technical production in the chemical industry is largely carried out via the gas phase oxidation of propane or propene in the presence of suitable heterogeneous catalysts . This partial oxidation on the solid contact (synonymous with “heterogeneous catalyst”) takes place with air as the oxidizing agent at temperatures around 330–390 ° C, using tube bundle reactors in which the strongly exothermic reaction is cooled with salt baths. Only a relatively dilute mixture of propene with air (usually still in the presence of water vapor) is used in order to avoid the formation of explosive mixtures. The yields that can be achieved with modern catalysts are up to over 80% with respect to propene, while by-products are around 5% acrylic acid and carbon monoxide and carbon dioxide in addition to unconverted propene. The quenching and isolation of the acrolein before further use as a raw material for the synthesis of methionine , glutaraldehyde or various fragrances is problematic . During isolation, acrolein and other active secondary components (in particular acrylic acid esters) polymerize , which force the system to be shut down.
In addition, acrolein is an undesirable product of many combustion and oxidation reactions of various organic compounds. When various organic substances (printer ink, vegetable oils, biodiesel, wax, tobacco, etc.) are burned, acrolein vapors are generated, for example in industry, with car exhaust fumes, cigarette smoke (up to 140 μg / cigarette). The typical acrole smell occurs immediately after a candle has gone out. It is also produced when vegetable and animal fats are overheated, for example when deep-frying . This process is made much easier by the presence of water and acids. The fat ( triglyceride ) is first broken down into its components glycerine and fatty acids ( hydrolysis ). The glycerine is then converted into acrolein by splitting off water ( dehydration ).
Attempts are increasingly being made to stop producing acrolein from propane or propene. Propane and propene are petrochemical products and are based on natural gas or crude oil . These fossil raw materials will become increasingly scarce in the future. That is why many companies try to obtain acrolein by dehydrating glycerin . This occurs as a cheap by-product in the production of biodiesel from natural fats and oils.
properties
The small size of acrolein, the aldehyde group and the double bond that is present ensure that the molecule is highly reactive. In its pure form, acrolein is hardly stable.
Because of the C = C double bond present , acrolein can polymerize easily. Acrolein is also converted into glycerine by adding water .
The aldehyde group can bind to proteins . Acrolein is therefore used for fixation in electron microscopy , similar to formalin . The advantage lies in the particularly short reaction time compared to other aldehydes.
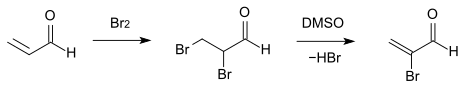
Acrolein reacts with elemental bromine to give the corresponding 2,3-dibromo compound, with dimethyl sulfoxide selectively to the 2-bromoacrolein dehydrohalogenated can be.
hazards
Acrolein is very toxic and also a strong environmental toxin . It is a powerful water and marine pollutant and very harmful to fish. According to the MAK commission of the DFG, acrolein is carcinogenic (category 3B). This category includes substances that give cause for concern because of their proven / possible carcinogenic effects.
Acrolein is very flammable and can form explosive mixtures with the air.
Metabolic formation from the cytostatic drug cyclophosphamide
The cytostatic agent cyclophosphamide is metabolized in the liver, resulting in a non-enzymatic cleavage into the actual active ingredient chloroethylphosphoric acid amide and the by-product acrolein. Acrolein has urotoxic effects.
use
Acrolein is used as an intermediate in the large-scale production of the synthetic amino acid DL - methionine and its hydroxy analogue DL -2-hydroxy-4-methylmercaptobutyric acid , which are of considerable economic importance as a compound feed component in animal nutrition .
Acrolein is mainly used as an intermediate in the synthesis of chemical compounds (such as acrylic acid and β-hydroxypropionaldehyde) and as a biocide . It is also produced when certain pollutants are broken down in the outside air or when organic substances, including tobacco or fuels such as gasoline or oil, are burned. Acrolein-containing emissions can be measured using the 2-HMP method .
Individual evidence
- ↑ a b c Entry on acrolein. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b c d e f g h Entry on acrylaldehyde in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-8.
- ↑ Entry on acrylic aldehyde in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 107-02-8 or acrolein ), accessed on November 2, 2015.
- ↑ Patent DE4023239A1: Patent process for the catalytic gas phase oxidation of propene or isobutene to acrolein or methacrolein - Google patent search , accessed on February 6, 2017
- ↑ Houben-Weyl Methods of Organic Chemistry Vol. E 3, 4th Edition Supplement Aldehydes . Georg Thieme Verlag, 2014, ISBN 978-3-13-181134-9 , p. 234 ( limited preview in Google Book search).
- ↑ Dissertation Vanessa Lehr: Dehydration of glycerol to acrolein , Technical University Darmstadt, 2008.
- ↑ W. Li, J. Li, Z.-K. Wan, J. Wu, W. Massewski: Preparation of α-Haloacrylate Derivatives via Dimethyl Sulfoxide-Mediated Selective Dehydrohalogenation . In Org. Lett 2007 , 9 , pp. 4607-4610. doi : 10.1021 / ol7021142
- ↑ Entry on methionine. In: Römpp Online . Georg Thieme Verlag, accessed on June 21, 2016.
- ↑ Acrolein data sheet from Sigma-Aldrich , accessed on March 7, 2011 ( PDF ).
- ↑ US EPA: Acrolein , accessed April 16, 2015.
- ↑ VDI 3862 sheet 5: 2008-06 measurement of gaseous emissions; Measurement of lower aldehydes especially acrolein with the 2-HMP method - GC method (Gaseous emission measurement; Measurement of lower aldehydes especially acrolein with the 2-HMP method - GC method). Beuth Verlag, Berlin, p. 4.
Web links
- Entry on acrolein . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD .