Sohio process
The Sohio process is a chemical-technical process for the production of acrylonitrile and acrolein . It was developed by the company of the same name, Sohio . After it was taken over by BP , the systems continued to be operated and continuously improved. The syntheses take place in the context of a catalytic oxidation of propene . The process used to make acrylonitrile is actually the largest industrial ammoxidation process .
Historical development

In 1953, the search for a cheap process began to produce aldehydes from simple aliphatic hydrocarbons, which was very expensive until then. Propane and propene were considered as starting materials . Franklin Vaeth had the idea of converting propene with a metal oxide catalyst . Two years later there was no significant success and Sohio gave Veath 6 weeks. Within this time, however, the desired reaction actually succeeded with the help of a modified vanadium pentoxide catalyst. The product that resulted was acrolein. This is converted into acrylic acid, which was previously a very expensive substance, through a further oxidation process.
So in 1955, a further test phase was initiated in which Jim Callahan and Emily Ross found a bismuth phosphomolybdate catalyst that laid the foundation for the Sohio process. Jim Idol suspected that acrylonitrile can even be obtained directly with this catalyst without an additional step. The process was developed in 1957 by him and Evelyn Jonak. Since a synthesis attempt already gave a yield of 50% and the production of acrylonitrile and acrolein was too complicated at the same time, the process for the synthesis of acrylonitrile was first developed.
Synthesis of acrylonitrile
The technical realization is considered on the schematic representation of a plant for the production of acrylonitrile.
Technical realization
| Structural formula | Surname | Molecular formula | Boiling temperature [in ° C] |
|---|---|---|---|
| Acetonitrile | C 2 H 3 N | 82 | |
| Acrylonitrile | C 3 H 3 N | 77 | |
| Prussic acid | HCN | 26th | |
| water | H 2 O | 100 |
The large-scale production of acrylonitrile is operated with a plant that can be combined into two areas. In the first area, acrylonitrile is synthesized with the byproducts hydrogen cyanide (HCN) and acetonitrile (C 2 H 3 N) and then separated in the second part.
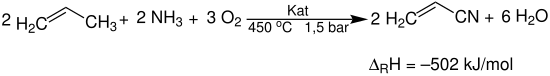
Initially, in the fluidized bed reactor 1 propene, ammonia and air is added to a fluidized fluidised bed of the catalyst. The reaction is strongly exothermic. To keep the temperature in the reactor constant, it must be permanently cooled with water . The main product acrylonitrile is formed according to the following reaction equation:
Then, in the second part of the system, the product gases with the remaining components of the air are passed into the absorber 2 , where they are separated from one another. What remains is a mixture of water, acrylonitrile, hydrocyanic acid and acetonitrile , which are fed into the distillation column 3 . This creates two different fractions. The first fraction (acetonitrile and water) are passed into the distillation column 4b and separated from one another there. The acetonitrile can be collected as a liquid and the water returns to the absorber 2 . The second fraction (acrylonitrile and hydrocyanic acid) is passed into the distillation chamber 4a and separated there from one another.
Catalyst development
Over the years, it was imperative to develop the catalyst. At the beginning of development, the Sohio process only yielded yields of around 50 percent based on acrylonitrile. Further research aimed at reducing the proportion of the byproducts hydrogen cyanide and acetonitrile. From 1960, bismuth-phosphomolybdate contacts were used for production. A uranyl antimonate catalyst was developed in 1967 to reduce the proportion of acetonitrile. In 1972 the so-called "Catalyst 41" was used for the first time, which is an iron-modified bismuth molybdate catalyst and keeps the proportion of both hydrogen cyanide and acetonitrile low.
Selectivity and economic importance
If 100 grams of propene are used in this process, the following optimized yields result based on the amount of propene used:
| Surname | Mass [in g] | Amount of substance [in mol] | Yield [in%] |
|---|---|---|---|
| Acrylonitrile | 90 | 1.70 | 71.7 |
| Acetonitrile | 2 | 0.05 | 2.1 |
| Prussic acid | 15th | 0.55 | 23.2 |
The economic importance of this process is very high. About 90% of the global demand for acrylonitrile is produced using this process. The company INEOS Köln GmbH (former BP and even earlier Bayer Erdölchemie) even operates several plants at its location in Cologne.
The world annual production in 1991 was 4.4 million tons.
Synthesis of acrolein
The historical development has already shown that acrolein can also be synthesized using the Sohio process. The development of the catalyst for the synthesis of acrylonitrile also achieved the economic breakthrough for acrolein production. Here a partial gas phase oxidation of propene takes place in the presence of suitable heterogeneous catalysts according to the following reaction equation:
This partial oxidation on the heterogeneous catalyst takes place with air as the oxidizing agent at temperatures around 330-390 ° C, with tube bundle reactors being used, in which the strongly exothermic reaction is cooled with salt baths. Only a relatively dilute mixture of propene with air is used, usually still in the presence of water vapor. This prevents the formation of explosive mixtures. The yields that can be achieved with modern catalysts are up to over 80% with respect to propene. The by-products are around 5% acrylic acid , carbon monoxide and carbon dioxide , in addition to unreacted propene.
Individual evidence
- ^ A b c Klaus Weissermel , Hans-Jürgen Arpe : Industrial Organic Chemistry, 5th Edition, Wiley-VCH Verlag GmbH, Weinheim 1998, ISBN 3-527-28856-2 , pp. 335-336.
- ↑ Historical overview of the development of the Sohio process .
- ^ Wilhelm Keim, Arno Behr, Günter Schmitt: Basics of Industrial Chemistry, 1st edition, Otto Salle Verlag GmbH & Co., Frankfurt am Main, Verlag Sauerländer AG, Aarau 1986, ISBN 3-7935-5490-2 (Salle), ISBN 3-7941-2553-3 (Sauerländer), pp. 314-315.
- ↑ a b c d Ulfert Onken, Arno Behr: Chemical Process Studies - Textbook of Technical Chemistry , Volume 3, 1st Edition, Georg Thieme Verlag, Stuttgart 1996, ISBN 3-13-687601-6 , pp. 315-317.
- ↑ Patent DE4023239A1: Patent process for the catalytic gas phase oxidation of propene or isobutene to acrolein or methacrolein - Google patent search , accessed on February 6, 2017.
Web links
- Technical illustration , from page 33
- Fluidization engineering , by Daizō Kunii, Octave Levenspiel
- INEOS GmbH production scheme for its Cologne location