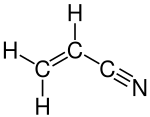
Acrylonitrile
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Acrylonitrile | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 3 N | ||||||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 53.06 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.8 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−82 ° C |
||||||||||||||||||
| boiling point |
77 ° C |
||||||||||||||||||
| Vapor pressure |
117 h Pa (20 ° C) |
||||||||||||||||||
| solubility |
moderate in water (73 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.3911 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 2 ml m −3 or 4.5 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
147.1 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Acrylonitrile ( IUPAC prop-2-enenitrile ) is the nitrile of acrylic acid . It is the simplest unsaturated nitrile.
Technical manufacturing
Acrylonitrile is produced using the Sohio process , a catalytically controlled conversion of propene with ammonia and pure oxygen . The reaction is also known as ammoxidation of propene. This produces acrylonitrile with elimination of water with acetonitrile and hydrocyanic acid as by-products . The catalyst is a mixture of iron , bismuth and molybdenum .
use
Acrylonitrile is used as a raw material for the production of acrylic acid , acrylic esters and acrylamide . It is also a component for adhesives , antioxidants , emulsifiers and solvents . The most important use of acrylonitrile is the polymerization to polyacrylonitrile and other copolymers ( acrylonitrile-butadiene-styrene (ABS) , styrene-acrylonitrile (SAN) , acrylonitrile-butadiene-rubber (NBR) and acrylic ester-styrene-acrylonitrile (ASA) ). In organic syntheses it is used for cyanoethylation .
Emission measurement
The emission measurement of exhaust gas loaded with acrylonitrile is usually carried out by gas chromatography . Sampling can be carried out using a gas collection vessel or absorption in cryogenic solvents. The draft of a VDI guideline , which describes the measurement of acrylonitrile by adsorption on activated carbon and desorption by dimethylformamide , was withdrawn in 2004.
Individual evidence
- ↑ a b Entry on acrylonitrile. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ a b c d e f g Entry on acrylonitrile in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-8.
- ↑ Entry on acrylonitrile in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 107-13-1 or acrylonitrile ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ^ History of the development of the process ( memento of October 12, 2006 in the Internet Archive ) at Sohio .
- ↑ VDI 3863 sheet 1: 1987-04 measurement of gaseous emissions; Measuring acrylonitrile; Gas chromatographic method; Sampling with gas collection vessels (measurement of gaseous emission; determination of acrylonitrile; gaschromatographic method; grab sampling). Beuth Verlag, Berlin, p. 2.
- ↑ VDI 3863 sheet 2: 1991-02 measurement of gaseous emissions; Measuring acrylonitrile; Gas chromatographic process; Sampling by absorption in low temperature solvents (Gaseous emission measurement; determination of acrylonitrile; gas chromatographic method; sampling by absorption in low temperature solvents). Beuth Verlag, Berlin, p. 2.
- ^ Association of German Engineers : VDI guideline: VDI 3863 Part 3 Measurement of gaseous emissions; Measuring acrylonitrile; Adsorption on activated carbon; Desorption by dimethylformamide (DMF) , accessed July 13, 2017




