Polyacrylonitrile
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
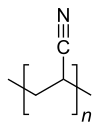
|
|||||||
| General | |||||||
| Surname | Polyacrylonitrile | ||||||
| other names |
PAN |
||||||
| CAS number | 25014-41-9 | ||||||
| Monomer | Acrylonitrile | ||||||
| Molecular formula of the repeating unit | C 3 H 3 N | ||||||
| Molar mass of the repeating unit | 53.06 g mol −1 | ||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.14-1.18 gcm -3 at 20 ° C |
||||||
| Melting point |
300 ° C |
||||||
| Glass temperature |
105 ° C |
||||||
| solubility |
soluble in strong bases and highly polar organic solvents such as DMSO , DMF , DMAC |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polyacrylonitrile ( abbreviated PAN ) is the polymer of acrylonitrile .
Structure and properties
As a pure substance, the polymer is hard, stiff, resistant to chemicals and solvents and has a melting point above the decomposition temperature. It is mostly produced by means of radical polymerisation .
history
Polyacrylonitrile (PAN) was first synthesized in 1930 by Hans Fikentscher and Claus Heuck in the Ludwigshafen plant of what was then IG Farben . Since PAN was infusible and insoluble in the solvents common at the time, the substance - similar to the polymers of tetrafluoroethylene at IG Farben in Höchst - was not investigated further as a material. The chemist Herbert Rein (1899–1955), who worked at the IG Farben plant in Bitterfeld , received a sample of the material during a visit to Ludwigshafen in 1931 and found the first good solvent for PAN in 1934 with pyridinium benzyl chloride - an ionic liquid . In 1942, Rein discovered that PAN is also readily soluble in dimethylformamide and, based on this, developed a technical processing process for spinning PAN fibers. After the war, the large-scale production of PAN as "Orlon" was initially started at DuPont in the USA.
use
The main use is in textile fibers ("polyacrylic"), consisting of copolymers , which are usually made of acrylonitrile (share> 85%) and one or more comonomers, such as. B. consist of methyl methacrylate . These fibers have been and are under various brands , for example
- Dralon (formerly Bayer AG , today Dralon),
- Dolan (formerly Hoechst AG , today Dolan GmbH),
- Orlon (formerly DuPont , now Invista),
- Crylor ( Radici Partecipazioni ) or formerly marketed as Wolpryla in the GDR .
The fibers are mostly textured and therefore have a high degree of bulk, which means that the textiles have a wool-like character and are warm, soft and crease- resistant . That is why polyacrylic is used in sweaters, imitation fur and blankets, often mixed with cotton or wool , but also processed alone.
In addition, PAN is used in other copolymers, e.g. B. together with polyvinyl chloride (PVC) for flame-retardant fibers (e.g. for monofilament synthetic hair, sold as Kanekalon) or together with 1,3-butadiene and styrene as acrylonitrile-butadiene-styrene copolymer (ABS).
It is also used for high-tensile, low-stretch synthetic ropes. PAN is used as a support layer in membrane technology.
PAN is also the most important raw material for the production of carbon fibers .
Thin fibers made from modified PAN (esterification and further oxidation to acid endings .. PAN-COOH) can be stimulated to muscle-like contractions by alternating rinsing with sodium hydroxide and hydrochloric acid . The force developed depends on the degree of polymerisation, the concentration of the rinsing liquids and the mechanical density of the fiber felt.
A disadvantage of the plastic is the formation of hydrogen cyanide in smoldering fires or in high heat.
Use of textile fiber
In addition to some advantages such as good dyeability, the textile products require compliance with a few care instructions . The fiber is sensitive to heat and may only be washed at a maximum of 40 ° C and not ironed while hot (only level 1). It is better to avoid tumble drying. A chlorine bleach is not possible. Cationic dyes are used for coloring .
literature
- Hans Domininghaus: Plastics. Properties and uses. 8th, revised and expanded edition. Springer, Berlin Heidelberg 2012, ISBN 978-3-642-16172-8 .
Individual evidence
- ↑ Thomas Gries, Dieter Veit, Burkhardt Wulfhorst: Textile manufacturing processes - An introduction. 2nd, revised and expanded edition. Carl Hanser Verlag, Munich 2014, ISBN 978-3-446-44057-9 , p. 70.
- ↑ a b c VA Bhanu, P. Rangarajan, K. Wiles, M. Bortner, M. Sankarpandian, D. Godshall, TE Glass, AK Banthia, J. Yang, G. Wilkes: Synthesis and characterization of acrylonitrile methyl acrylate statistical copolymers as melt processable carbon fiber precursors . In: polymer . tape 43 , no. 18 , August 2002, p. 4841-4850 , doi : 10.1016 / S0032-3861 (02) 00330-0 (English, researchgate.net [PDF]).
- ↑ Menachem Lewin (Ed.): Handbook of Fiber Chemistry. 3. Edition. Taylor & Francis Group, Boca Raton 2007, ISBN 978-0-8247-2565-5 , p. 915.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ polyacrylonitrile. chemie.fu-berlin.de, accessed on December 12, 2009 .
- ↑ Patent DE654989 : Process for the production of polymerization products. Registered on February 18, 1930 , published December 16, 1937 , inventors: H. Fikentscher, C. Heuck.
- ↑ Walter Wetzel: History of the discovery of polyfluoroethylene . In: NTM International Journal of History and Ethics of Natural Sciences, Technology and Medicine . tape 13 , no. 2 , May 2005, p. 79 , doi : 10.1007 / s00048-005-0210-x .
- ↑ The salt of fashion . In: Der Spiegel . No. 20 , 1955, pp. 16-18 ( online ).
- ↑ Patent DE631756 : Process for dissolving polymeric acrylonitrile. Registered August 8, 1934 , published June 4, 1936 , inventor: H. Rein.
- ↑ Patent DE763277 : Process for the production of threads from films of plastics. Registered June 10, 1942 , published December 28, 1944 , inventor: H. Rein.
- ↑ Information on the Dralon brand in the register of the German Patent and Trademark Office (DPMA)
- ↑ Information on the Dolan brand in the register of the German Patent and Trademark Office (DPMA)
- ↑ Information on the Orlon brand in the register of the German Patent and Trademark Office (DPMA)
- ↑ Crylor and information on the Wolpryla brand in the register of the German Patent and Trademark Office (DPMA)

