Styrene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Styrene | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 8 H 8 | ||||||||||||||||||
| Brief description |
colorless, sweet-smelling, strongly refractive liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 104.15 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.91 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−31 ° C |
||||||||||||||||||
| boiling point |
145 ° C |
||||||||||||||||||
| Vapor pressure |
7.14 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5458 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Styrene (also vinylbenzene , according to the IUPAC nomenclature phenylethene / ethenylbenzene ) is an unsaturated , aromatic hydrocarbon. It is a colorless, low-viscosity and sweet-smelling liquid. Styrene is flammable and harmful.
Styrene is an easily polymerizable , important monomer for the production of plastics such as polystyrene as well as styrene-acrylonitrile , acrylonitrile-butadiene-styrene and other copolymers . The chemical industry worldwide produced around 25 million tons of styrene in 2010.
history
The Berlin pharmacist Eduard Simon acquired Styrax , the resin of the oriental sweetgum tree ( Liquidambar orientalis ), which grows in the Near East , around 1835 . This tree resin was added to perfumes and medicines by the ancient Egyptians. While distilling this tree wax, he discovered a colorless liquid and named it after the raw material styrene. As he warmed the liquid, a new substance formed. He assumed it was styrene oxide .
However, the English chemist John Blyth and the German chemist August Wilhelm von Hofmann found out through elemental analysis in 1845 that the material composition had not changed. Marcelin Berthelot correctly interpreted the change in heating in 1866 as polymerisation. Hermann Staudinger , who mainly dealt with polymer chemistry , finally described in his theses that the warming starts a chain reaction in which the macromolecules of the polystyrene are formed.
Around 1930 the development of technical processes for styrene production began. Before that, it had to be isolated from the pyrolysis gasoline . During the Second World War , the demand increased because it was needed for the styrene-butadiene copolymer . After the Second World War it was synthetically produced in larger quantities because of the high demand for polystyrene.
Occurrence
Styrene occurs in small amounts in Styrax ( tree resin ), in coal tar and in pyrolysis products of petroleum (around 7% in cracked gasoline ). In the last two decades there has been an increase in styrene emissions due to the increased use of styrene plastics. In nature, styrene is a flavoring substance and occurs, for example, in small quantities in grapes and kiwis , and it is also contained in the scent of orchid flowers . Little is known about the effect; it probably serves to attract pollinators in the case of flowers or consumers to distribute seeds on fruits. More recent research has shown that styrene can also be produced during the brewing process of wheat beer .
Manufacturing
There are currently two processes of industrial importance for the production of styrene, the dehydrogenation of ethylbenzene and the SM / PO process. In 2012 the annual world production of styrene was about 20 million tons.
Dehydrogenation of ethylbenzene
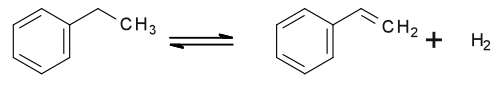
Styrene is produced on an industrial scale from ethylbenzene . This is obtained by distillation from the BTEX fraction of pyrolysis gasoline or by a Friedel-Crafts or zeolite-catalyzed gas or liquid-phase alkylation of benzene with ethene . Ethylbenzene is used almost exclusively to make styrene.
The most common process here is the catalytic dehydrogenation of ethylbenzene. For this purpose, ethylbenzene is dehydrated with a ten to fifteen-fold excess of high-pressure steam over an alkaline iron (II) oxide contact.
The contact usually contains a few percent sodium oxide or sodium carbonate . The steam serves as a supplier of the required heat of reaction and reacts with the coke deposited on the catalyst to form carbon monoxide and hydrogen and further in a water gas shift reaction to form carbon dioxide and hydrogen.
Industrial plants mostly consist of a series of fixed bed reactors, with conversions of 70 to 75% with selectivities of 93 to 97% for styrene. The crude product is separated from the by-products such as toluene and higher alkylated benzenes and the unreacted benzene by distillation . Free radical scavengers such as nitrated phenols are used to avoid styrene polymerization .
Shell SMPO process
In the indirect propene (Shell SMPO method = Styrene monomer & Propylene Oxide) is ethylbenzene at approximately 2 bar and 150 ° C to ethyl benzene peroxidized. The ethylbenzene hydroperoxide epoxidizes propene at high pressure and 115 ° C on titanium on silicon dioxide to propylene oxide . This produces 1-phenylethanol . This is dehydrated to styrene at around 200 ° C over aluminum oxide . In Germany, only about 10% of the styrene produced is made by this process.
Other procedures
In addition, there are processes to recover styrene from polystyrene products, sometimes from napalm-B . Styrene can be made by alkylating toluene with methanol . The raw materials used are usually cheaper than those used in the dehydrogenation of ethylbenzene, but the selectivity for the target product is lower. Further synthesis routes are the dimerization of 1,3-butadiene to 4-vinylcyclohexene and subsequent dehydrogenation and the tetramerization of ethyne as a by-product of the cyclooctatetraene synthesis according to Walter Reppe .
Laboratory syntheses
In the laboratory, styrene can be prepared by dry distillation of cinnamic acid with decarboxylation . It is the first historical representation method.
properties
Physical Properties
Styrene is only very slightly soluble in water at 240 mg / l at 20 ° C , and is readily soluble in acetone , ether , carbon disulphide , dichloromethane and alcohol . With a refractive index of 1.5458, styrene refracts light even more than benzene. The flash point is 31 ° C, the ignition temperature is 490 ° C. The viscosity is 0.7 mPas (20 ° C). The energy of the conjugation of the double bond of the vinyl group with the benzene ring is about 29 kJ / mol and is thus higher than that of the conjugation of a double bond system.
Chemical properties
Styrene has a pleasantly sweet smell , the odor threshold is between 0.43 and 866 mg · m −3 (according to the material data sheet: 0.02–3.4 mg · m −3 ). Styrene polymerizes to a yellow, sticky liquid at room temperature, which is why it is stabilized with up to 50 ppm of 4- tert- butylpyrocatechol or hydroquinone . The stabilizers only work in the presence of small amounts of oxygen, since they initially act as antioxidants, the products formed during the oxidation of the stabilizers act as inhibitors . When exposed to light, oxygen or heat, the polymerization is significantly accelerated, so it is stored in dark glasses and in cooler places.
| Relative arrangement of the phenyl residues in the polystyrene |
|---|
 Atactic polystyrene |
 Syndiotactic polystyrene |
 Isotactic polystyrene |
The polymerisation properties of styrene are extraordinary: it includes thermal, radical, coordinative, anionic and cationic polymerisation. For example, iron chloride can be added to the styrene for cationic polymerization. If the styrene is brought to the boil with the help of a Bunsen burner , it can be removed from the flame and it will continue to react to form polystyrene on its own . In thermal polymerization, styrene is simply exposed to heat. In radical polymerization, a radical combines with the styrene, making the styrene itself a radical. Organic peroxides such as dibenzoyl peroxide are often used as radical initiators . These styrene radicals can polymerize. At the end two radicals combine again ( chain termination ). Depending on the catalyst, atactic , syndiotactic or isotactic polystyrene can be obtained. The heat of polymerization is −70 kJ mol −1 or −670 kJ kg −1 .
The reaction behavior of styrene is characterized by the competition between the vinyl group and the aromatic ring. Therefore, in many cases it reacts differently than, for example, benzene. Some electrophiles, which tend to undergo addition reactions , and radicals preferentially attack the vinyl group, since there is no need to break the aromatic state in the transition state of the reaction, as is the case with attack on the ring. Typical electrophilic substitution reagents , which usually do not add to alkenes , substitute the styrene on the ring. Examples of this are nitration and sulfonation. With halogens, however, the addition to the vinyl group is significantly faster than the substitution reaction to the aromatic.
The vinyl group reacts with peroxycarboxylic acids in an electrocyclic reaction to form styrene oxide .
Safety-related and environmental parameters
Styrene forms highly flammable vapor-air mixtures. The compound has a flash point of approx. 32 ° C. The explosion range is between 0.97% by volume (42 g / m 3 ) as the lower explosion limit (LEL) and 7.7% by volume (334 g / m 3 ) as the upper explosion limit (UEL). The maximum explosion pressure is 7.5 bar. The ignition temperature is 490 ° C. The substance therefore falls into temperature class T1.
Styrene is hazardous to water ( WGK 2 ), but it is biodegradable over a long period of time.
use

Styrene is processed into many plastics . Polystyrene is an important one . In 1997, 0.66 million tons of polystyrene were produced from styrene, so over 60% of the styrene was processed into polystyrene, making polystyrene one of the most important plastics of all. In addition to PU foams, polystyrene in foamed form is used as the most important thermal insulation material in the building materials industry and as transport protection for industrial and consumer goods.
Copolymers of styrene (polymer made from several monomers ) are, for example, acrylonitrile-butadiene-styrene (ABS), styrene-acrylonitrile (SAN), styrene-butadiene (SB) and acrylonitrile-styrene-acrylic ester (ASA). Styrene is also an important raw material in the manufacture of unsaturated polyester resins (UP resins). This contains 50–70% of it to polymerize together with the maleic acid-containing polyesters during curing (copolymerization).
toxicology
Styrene is absorbed through the respiratory organs and less through the skin and is mainly deposited in the liver , kidneys , brain and in adipose tissue . It irritates the respiratory tract, skin, eyes and mucous membranes. Inhalation and swallowing can lead to unspecific symptoms such as poor concentration, tiredness, nausea, dizziness, headaches and states of agitation. Styrene is excreted in the urine after about half a day. Occupational exposure can lead to changes in the semen analysis of men . Styrene can lengthen the time to a desired pregnancy. The occupational exposure limit is 20 ml / m 3 . Styrene is believed to be ototoxic in humans.
literature
- Advisory committee for environmentally relevant waste materials (BUA) of the German Chemical Society (Ed.): Styrene (ethenylbenzene). Wiley / VCH, Weinheim 1990, ISBN 3-527-28255-6 ( Gesellschaft Deutscher Chemiker. BUA-Stoffbericht 48).
Individual evidence
- ↑ Entry on STYRENE in the CosIng database of the EU Commission, accessed on May 17, 2020.
- ↑ a b c d e f g h i j k l m n o Entry on styrene in the GESTIS substance database of the IFA , accessed on April 16, 2015(JavaScript required) .
- ↑ a b c Entry on styrene. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ Entry on styrene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 100-42-5 or styrene ), accessed on November 2, 2015.
- ↑ New Process for Producing Styrene Cuts Costs, Saves Energy, and Reduces Greenhouse Gas Emissions, at eere.energy.gov. (PDF; 282 kB) Retrieved October 18, 2013 .
- ^ A b John Blyth, August Hofmann: About styrene and some of its decomposition products. In: Justus Liebig's Annals of Chemistry . 53.3, 1845, pp. 289-329, doi: 10.1002 / jlac.18450530302 .
- ↑ H. Staudinger, A. Steinhofer: About high polymer compounds. Part 107. Contributions to the knowledge of polystyrenes. In: Justus Liebig's Annals of Chemistry . 517, 1935, pp. 35-53, doi: 10.1002 / jlac.19355170104 .
- ↑ KJ Schwarz, R. Stübner, FJ Methner: Formation of styrene dependent on fermentation management during wheat beer production. In: Food Chem. 134 (4), Oct 15, 2012, pp. 2121-2125. PMID 23442664 .
- ↑ Fangfang Wang, Yajun Wang: Safety Assessment of Production Process of Styrene. In: Procedia Engineering . 45, 2012, pp. 139-143, doi: 10.1016 / j.proeng.2012.08.134 .
- ↑ G. Bellussi: Liquid-Phase Alkylation of Benzene with Light Olefins Catalyzed by β-Zeolites. In: Journal of Catalysis . 157, 1995, pp. 227-234, doi: 10.1006 / jcat.1995.1283 .
- ↑ M. Muhler: The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene 2. Surface chemistry of the active phase. In: Journal of Catalysis . 138, 1992, pp. 413-444, doi: 10.1016 / 0021-9517 (92) 90295-S .
- ↑ Ailing Sun, Zhangfeng Qin, Jianguo Wang: Reaction coupling of ethylbenzene dehydrogenation with water-gas shift. In: Applied Catalysis A: General . 234, 2002, pp. 179-189, doi: 10.1016 / S0926-860X (02) 00222-3 .
- ↑ JKF Buijink, JJM van Vlaanderen, M. Crocker, FGM Niele: Propylene epoxidation over titanium-on-silica catalysts - the heart of the SMPO process. In: Catalysis Today . 93-95, 2004, pp. 199-204, doi: 10.1016 / j.cattod.2004.06.041 .
- ↑ Patent US5406010 - Method of reclaiming styrene and other products from polystyrene based products. Retrieved June 1, 2013 .
- ↑ Tatsuaki Yashima, Keiichi Sato, Tomoki Hayasaka, Nobuyoshi Hara: Alkylation on synthetic zeolites: III. Alkylation of toluene with methanol and formaldehyde on alkali cation exchanged zeolites. In: Journal of Catalysis . , 26, 1972, pp. 303-312, doi: 10.1016 / 0021-9517 (72) 90088-7 .
- ↑ GN Schrauzer: coordination chemistry and catalysis studies. About the cyclooctatetraene synthesis according to W. Reppe. In: Angewandte Chemie . 76, 1964, pp. 28-35, doi: 10.1002 / anie.19640760105 .
- ↑ R. Fittig, F. Binder: Ueber die additionproducte der Zunftssaure. In: Rudolph Fittig, Camille Petri: Investigations on the unsaturated acids. I. Further contributions to the knowledge of fumaric acid and maleic acid. In: Justus Liebig's Annals of Chemistry . Volume 195, 1879, pp. 56-179, doi: 10.1002 / jlac.18791950103 .
- ↑ L. Pauling : The nature of the chemical bond. Verlag Chemie, Weinheim 1973, ISBN 3-527-25217-7 , p. 276.
- ↑ Employer's liability insurance association for raw materials and chemical industry , leaflet R 008 Polyreactions and polymerizable systems. Edition 05/2015, ISBN 978-3-86825-069-5 .
- ↑ a b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ a b M. Roller: Work-related disorders of reproduction when handling hazardous substances - an information document. Federal Institute for Occupational Safety and Health, Project No. F 1925: Reproductive disorders due to hazardous substances in the workplace.
- ↑ P. Hoet, D. Lison: Ototoxicity of toluene and styrene: state of current knowledge. In: Crit Rev Toxicol. Volume 38, Issue 2, 2008, pp. 127-170. doi: 10.1080 / 10408440701845443 . PMID 18259983 .