Iron (II) oxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
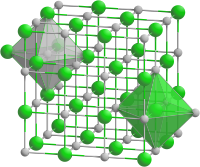
|
|||||||||||||||||||
| __ Fe 2+ __ O 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Iron (II) oxide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | FeO | ||||||||||||||||||
| Brief description |
black powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 71.85 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
5.75 g cm −3 |
||||||||||||||||||
| Melting point |
1369 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
1.5 mg m −3 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Iron (II) oxide (formerly also called iron oxide ) is a chemical compound of iron and oxygen and is one of the oxides . Iron (II) oxide is normally not built up stoichiometrically , the composition is around Fe 0.84 O to Fe 0.95 O. The reason for this is the similar crystal structure of iron (III) oxide and that crystal defects are entropically favorable.
Occurrence
Iron (II) oxide occurs during the fresh process / LD process (lowering the carbon content ) in steel production . Here, oxygen is blown into the iron melt. The oxygen combines with iron to form FeO, which in turn acts as an oxidizing agent for interfering foreign elements such as silicon , manganese and phosphorus :
With the help of calcium oxide (CaO) these oxides can be separated from the iron melt.
In nature, iron (II) oxide occurs as a mineral wüstite .
Extraction and presentation
Iron (II) oxide is formed during the reduction of iron (III) oxide with hydrogen or carbon monoxide . Iron (II) oxide can also be obtained by oxidizing iron under low oxygen pressure or with water vapor at temperatures above 560 ° C.
Stoichiometric iron (II) oxide can be produced by heating iron (II) oxalate to around 850 ° C in a vacuum and then quickly quenching it to room temperature.
Furthermore, stoichiometric iron (II) oxide is formed in the reaction of Fe 1-x O and iron at 770 ° C. and 50 kbar oxygen pressure.
properties
Iron (II) oxide is only stable above 560 ° C. Below this temperature down to approx. 300 ° C it tends to disproportionate to iron and iron (II, III) oxide :
It is metastable at room temperature . It can be easily oxidized; finely divided FeO obtained from the oxalate by pyrolysis is pyrophoric .
Iron (II) oxide antiferromagnetic with a Néel temperature of 198 K .
Individual evidence
- ↑ Entry on CI 77489 in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b c d e Entry for CAS no. 1345-25-1 in the GESTIS substance database of the IFA , accessed on August 1, 2007 (JavaScript required)
- ↑ a b Data sheet Iron (II) oxide at Sigma-Aldrich , accessed on March 29, 2011 ( PDF ).
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1652.
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 .





