Oxalic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
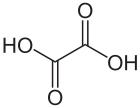
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Oxalic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 2 O 4 | |||||||||||||||||||||
| Brief description |
color- and odorless, crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| Sublimation point |
157 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
moderate in water (90–100 g · l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 1 mg m −3 (measured as inhalable dust ) |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−829.9 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Oxalic acid (systematic name: ethanedioic acid, historically: clover acid and acidum oxalicum) is the simplest dicarboxylic acid . Their salts are called oxalates (systematically: ethanedioate). Oxalic acid is a reducing agent and can therefore be determined quantitatively by titration with an oxidizing agent such as potassium permanganate , which produces carbon dioxide as an oxidation product .
history

Oxalic acid was discovered in 1769 by Johann Christian Wiegleb in wood sorrel ( Oxalis acetosella , hence the name) as a potassium salt and was therefore only known under the name of clover acid. 1776 could in large quantities by Carl Wilhelm Scheele and Torbern Bergman by oxidation of sugar with nitric acid are produced, which no synthesis, but the degradation of a natural substance was. (In this method is also the historical name sugar acid, which today glucaric is called back.) Of oxalic acid was then for the first time in 1824 by Friedrich Wohler made artificially from inorganic basic (synthesized by saponification of cyanogen , (CN) 2 ).
Occurrence
Oxalic acid and its salts are found in large quantities in rhubarb (180–765 mg / 100 g fresh weight, stems) and other knotweed plants ( polygonaceae ) such as B. Alpine dock and sorrel (830–1770 mg / 100 g), most of them in the leaf blades, which is why only the stalks of the rhubarb leaves are suitable for consumption after cooking. Also, star fruit ( Averrhoa carambola ) contain a lot of oxalic acid (40-1000 mg / 100 g fresh weight). In similar amounts but oxalic acid is also used in the eponymous sorrel ( Oxalis ), Mangold (110-940 mg / 100 g fresh weight), spinach (120-1330 mg / 100 g fresh weight), parsley (0-185 mg / 100 g fresh weight) Cocoa (338–480 mg / 100 g), chocolate (80–200 mg / 100 g) and beetroot (17–329 mg / 100 g fresh weight). Oxalic acid is also excreted by many fungi ; their production is promoted by the alkaline reaction of the nutrient solution.
Occurrence in food
| plant | Oxalic acid (g / 100 g) |
|---|---|
| Amaranth | 1.09 |
| asparagus | 0.13 |
| Green bean | 0.36 |
| Beetroot (leaves) | 0.61 |
| broccoli | 0.19 |
| Brussels sprouts | 0.36 |
| Cabbage | 0.10 |
| carrot | 0.50 |
| Swiss chard | 0.69 |
| manioc | 1.26 |
| cauliflower | 0.15 |
| celery | 0.19 |
| Chicory | 0.2 |
| chives | 0.148 |
| Leaf cabbage | 0.45 |
| coriander | 0.01 |
| Sweet corn | 0.01 |
| cucumber | 0.02 |
| aubergine | 0.19 |
| endive | 0.11 |
| garlic | 0.36 |
| Kale | 0.02 |
| Garden salad | 0.33 |
| okra | 0.05 |
| onion | 0.05 |
| parsley | 0.17 |
| parsnip | 0.04 |
| pea | 0.05 |
| paprika | 0.04 |
| potato | 0.05 |
| Purslane | 1.31 |
| radish | 0.48 |
| Rhubarb leaves | 0.52 |
| Turnip | 0.03 |
| spinach | 0.97 |
| Pumpkins | 0.02 |
| sweet potato | 0.24 |
| tomato | 0.05 |
| Turnip | 0.21 |
| Beet leaves | 0.05 |
| Watercress | 0.31 |
Extraction and presentation
Nowadays, oxalic acid is made by rapidly heating sodium formate to 360 ° C.
The sodium oxalate obtained is first converted into the poorly soluble calcium oxalate with calcium hydroxide :
The end product oxalic acid is released from this by adding sulfuric acid; Calcium sulphate is produced as a by-product :
The worldwide production of oxalic acid and its esters is 140,000 tons per year.
properties
Chemical properties
Oxalic acid is determined by the position adjacent to the carboxy groups of a strong acid . When heated above 150 ° C, it decomposes with the formation of carbon monoxide, carbon dioxide and water. The decomposition takes place in two steps via the formation of formic acid :
Similarly, in concentrated sulfuric acid , oxalic acid instantly breaks down into carbon monoxide , carbon dioxide, and water . Oxalic acid crystallizes from aqueous solutions with two molecules of crystal water to form oxalic acid dihydrate [(COOH) 2 · 2 H 2 O]. Oxalic acid and its soluble salts are harmful to health .
use
Oxalic acid can be used to remove rust stains or as a bleach .
In beekeeping , oxalic acid is used as a winter treatment to combat the varroa mite . It is 3.5% (calculated for oxalic acid dihydrate, the effective oxalic acid concentration is 2.5%) in an aqueous sugar solution (50% sucrose solution ) dripped onto the bees or sprayed at 3 % . In its crystalline form as a dihydrate, it is also used in tablet or powder form in so-called vaporizers . It sublimes as a fine precipitate in the beehive, where it is distributed by the workers. The vaporizer is a construction of a metal container filled with a few grams of oxalic acid dihydrate, which is usually fired from below by a tea light . The evaporator is expediently placed in an empty frame in the beehive that is placed on top of the beehive and separated from it by close-meshed plastic or metal fabric. By separating the bees, they cannot extinguish the tea light by flapping their wings. This form of treatment has not yet been approved in Germany.
In the analytical laboratory, the oxalic acid dihydrate is used as the basic titer substance for manganometry . Furthermore, it serves as a basic titer substance for the exact determination of the content of alkaline standard solutions , such as sodium hydroxide . Due to the formation of a hardly soluble calcium salt, it is also important for the gravimetric determination of calcium ions as calcium oxalate . In addition, oxalic acid is used for measuring of ammonia used in the outside air by connecting the inner tubes of so-called denuder be coated with oxalic acid, and the resulting reaction product is analyzed.
In the Fichtel Mountains , oxalic acid obtained from wood sorrel was used to bleach quartz (rock crystal), which occurs here mainly under the town of Weißenstadt .
Oxalic acid ( clover salt ) is used to polish marble to a shine .
In woodworking , oxalic acid serves as a milder bleaching agent (compared to hydrogen peroxide ) for wood and is mainly used to remove black stains that are caused by a reaction of tannic acids (constituents of wood) with metal, for example when wood containing tannic acid comes into contact with iron Tools.
In drug chemistry, oxalic acid is used as a salt former . The protonation of basic amines creates oxalates.
Biological importance
Oxalic acid and oxalates are absorbed through food and are formed as a metabolic product when amino acids and ascorbic acid are broken down . It is excreted in the urine. Depending on the food, 5 - 50% of the oxalic acid excreted in the urine comes from food. If more than 45 mg (= 0.5 mmol) is excreted within 24 hours, one speaks of hyperoxaluria . This increases the risk of the precipitation of poorly soluble calcium oxalate in the form of kidney stones . Oxalic acid is harmful to health in higher concentrations, but occurs in low concentrations in foods such as tea (especially black tea and peppermint tea , see also occurrence ) and in the roots and bark of numerous plants as insoluble calcium oxalate . Calcium oxalate is often produced in nature when plant cells die. It can be seen under polarized light in the form of bright, rectangular crystals (especially easy in brown onion skins ). Kidney stones usually consist of calcium oxalate and uric acid , but stone formation is prevented by citric acid , which occurs in fruits.
Since oxalic acid makes the absorption (absorption) of iron more difficult in the intestine, iron therapy, e.g. B. in the context of iron deficiency anemia , be reluctant to eat foods with a high content of oxalic acid and do not consume them at the same time as iron tablets. After ingestion of oxalic acid, the affected tissue is depleted of calcium, which in severe cases can damage the heart. After taking larger doses, symptoms of paralysis can occur; in any case (even with slight poisoning), kidney damage occurs due to clogged kidney tubules . The lowest (known) lethal dose in humans ( LD Lo , oral) is given as 600 mg per kg body weight.
Plants with high oxalic acid content (e.g. stump-leaved dock ) are also not digestible for grazing animals and are avoided. Since the oxalic acid does not disappear when haying , meadows that are heavily weedy are problematic for the hay harvest.
literature
- Heinz GO Becker: Organikum : basic organic-chemical internship . 23rd edition. Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32292-3 .
- Hans Günther Schlegel: General Microbiology . 8th edition. Thieme, Stuttgart / New York 2007, ISBN 978-3-13-444608-1 .
Web links
- Safety data sheet oxalic acid
- Understand chemistry: oxalic acid
Individual evidence
- ↑ Entry on OXALIC ACID in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b c d e f Entry on oxalic acid in the GESTIS substance database of the IFA , accessed on February 25, 2013(JavaScript required) .
- ↑ a b c W. Riemenschneider, M. Tanifuji: Oxalic Acid in Ullmann's Encyclopedia of Industrial Chemistry , 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 / 14356007.a18_247.pub2 .
- ↑ a b entry on oxalic acid. In: Römpp Online . Georg Thieme Verlag, accessed on April 25, 2015.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-406.
- ↑ a b chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF, 78 kB).
- ↑ Entry on Oxalic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 144-62-7 or oxalic acid ), accessed on November 2, 2015.
- ↑ a b Entry on oxalic acid in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-21.
- ↑ Lexicon entry from 1857 on "Kleesäure"
- ^ Burckhard Frank: 250 years of chemistry in Göttingen . In: Hans-Heinrich Voigt (Ed.): Natural sciences in Göttingen. A series of lectures . Vandenhoeck + Ruprecht Gm, Göttingen 1988, ISBN 3-525-35843-1 ( Göttinger Universitätsschriften . Volume 13), p. 72 ( limited preview in Google book search and limited preview in Google book search)
- ↑ testudowelt.de Lutz Prauser: Calcium nutrition (# 5): Calcium and oxalic acid - all rhubarb? , in TESTUDOWELT (portal for news and information from the turtle world), accessed on July 4, 2018
- ↑ Hans Günther Schlegel: General Microbiology . 8th edition. Thieme, Stuttgart / New York 2007, ISBN 978-3-13-444608-1 .
- ↑ C. Wehmer: About oxalic acid formation by fungi . In: Justus Liebigs Annalen der Chemie , 269, 2-3, 1892, pp. 383-389, doi: 10.1002 / jlac.18922690214 .
- ↑ All data not specifically annotated is from Agriculture Handbook No. 8-11, Vegetables and Vegetable Products , 1984 ( "Nutrient Data: Oxalic Acid Content of Selected Vegetables" . Ars.usda.gov)
- ^ GW Pucher, AJ Wakeman, HB Vickery: The organic acids of rhubarb ( Rheum hybridium ). III. The behavior of the organic acids during the culture of excised leaves . In: Journal of Biological Chemistry . 126, No. 1, 1938, p. 43.
- ↑ Karsten Münstedt: Handbook of healthy beekeeping. Lehmanns Media, 2013, ISBN 978-3-86541-555-4 , pp. 61-63.
- ↑ Sandra Bielmeier: Bees Basics. Gräfe und Unzer, 2016, ISBN 978-3-8338-4738-7 . P. 124.
- ↑ Mellifera e. V .: Oxalic acid spray method approved for Varroa treatment , accessed on August 17, 2018.
- ↑ VDI 3869 sheet 3: 2010-10 Measurement of ammonia in the outside air; Sampling with coated diffusion separators (denuders); Photometric or ion chromatographic analysis (Measurement of ammonia in ambient air; Sampling with diffusion separators (denuders); Photometric or ion chromatographic analysis). Beuth Verlag, Berlin, p. 9 / p. 16.
- ^ David Charlesworth: Furniture-making Techniques, Vol. 2 . Guild of Master Craftsmen Publications Ltd., Lewes (East Sussex, UK) 2001, ISBN 1-86108-295-9 . (Page 86)
- ^ Declan O'Donoghue: The Complete Book of Woodworking . Lyons Press, London (UK) 2001, ISBN 1-59228-177-X . (Page 94).
- ↑ Nicola Siegmund-Schultze: Chronic renal insufficiency: Even moderately increased oxalate in the urine could indicate the progress of the disease. In: Deutsches Ärzteblatt . tape 116 , no. 14 , 2019, pp. A-694 .
- ↑ Yakkyoku [Pharmacy] . Vol. 31, 1980, p. 959.








