Iron (II) oxalate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
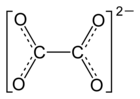
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Iron (II) oxalate | |||||||||||||||
| other names |
Ferro oxalate |
|||||||||||||||
| Molecular formula | FeC 2 O 4 | |||||||||||||||
| Brief description |
yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 143.85 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.28 g cm −3 (dihydrate) |
|||||||||||||||
| Melting point |
190 ° C (dihydrate, decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Iron (II) oxalate is an iron salt of oxalic acid .
Occurrence
Of course, iron (II) oxalate dihydrate occurs as a mineral Humboldtin (after Friedrich Heinrich Alexander von Humboldt).
Extraction and presentation
Iron (II) oxalate can be produced by reacting aqueous iron (II) salt solutions with oxalic acid or alkali metal oxalates.
properties
Iron (II) oxalate forms pale yellow, rhombic crystals. The dihydrate occurs in two different ( monoclinic and orthorhombic ) crystal forms.
If iron (II) oxalate is heated to over 190 ° C, the so-called Wüstit phase is obtained , a black iron oxide product that has a more or less large iron deficit compared to the formula FeO. Below is the equation of this reaction:
- .
- Iron oxalate disintegrates when heated to → iron (II) oxide + carbon monoxide + carbon dioxide
Under certain conditions, this reaction can also be used to produce stoichiometric iron (II) oxide ( see here ).
use
Iron (II) oxalate has been used as a developer in analog photography since 1879 . It is still used for optical glasses. It is also suitable for making pyrophoric iron.
literature
- R. Zboril, L. Machala et al. a .: Structural, magnetic and size transformations induced by isothermal treatment of ferrous oxalate dihydrate in static air conditions. In: physica status solidi. 1, 2004, p. 3583, doi: 10.1002 / pssc.200405511 .
- William Allen Miller: Elements of chemistry: theoretical and practical , Volume 2 ( limited preview in Google book search)
Individual evidence
- ↑ a b c d Data sheet iron (II) oxalate (dihydrate) from AlfaAesar, accessed on January 7, 2010 ( PDF )(JavaScript required) .
- ↑ tradekorea.com: Ferrous oxalate, battery grade , accessed March 3, 2019.
- ↑ a b Entry on iron (II) oxalate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ mineralienatlas.de: Humboldtin (mindat) .
- ↑ freepatentsonline.com: METHOD OF MANUFACTURING COPPER OXALATE .
- ↑ I. Sledzinska and A. Murasik: Nuclear and magnetic diffuse scattering of neutrons from β-ferrous oxalate dihydrate , in: Journal of Applied Crystallography , 1988 , 21 (5) , 504-511; doi: 10.1107 / S0021889888005758 .
- ↑ F. Aramu, V. Maxia, C. Muntoni: Mossbauer spectroscopy of polymorphous iron oxalate. In: Hyperfine Interactions. 5, 1977, p. 399, doi: 10.1007 / BF01021710 .
- ^ AF Holleman , E. Wiberg : Textbook of Inorganic Chemistry . 37-39 Edition. Walter de Gruyter, Berlin 1956, p. 534.
- ↑ Sir William De W. Abney: Instruction in Photography ( limited preview in Google book search)
- ↑ Schott: Spectroscopic Investigations .




