Iron (II, III) oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
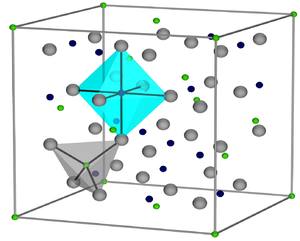
|
||||||||||||||||
| __ Fe 3+ __ Fe 2+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Iron (II, III) oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | Fe 3 O 4 | |||||||||||||||
| Brief description |
deep black, ferrimagnetic powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 231.54 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.2 g cm −3 |
|||||||||||||||
| Melting point |
1538 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Iron (II, III) oxide is an oxide of iron that contains both divalent and trivalent iron. It has the chemical formula FeO · Fe 2 O 3 or Fe 3 O 4 and is therefore also known as triiron tetraoxide . It is found in nature as magnetite . The melting point is 1538 ° C.
presentation
From iron and oxygen at very high temperatures:
Made of iron and water vapor at temperatures below 560 ° C
Made of iron (III) oxide at temperatures above 1200 ° C
From iron (III) oxide by reduction with hydrogen
As iron hammer or scale was formerly called the parachutist when forging hot iron iron particles immediately in air to Fe 3 O 4 oxidized.
Sinter is another name for the iron oxides that occur in continuous casting in the manufacture of steel slabs and in the hot rolling of steel. When the hot steel comes into contact with atmospheric oxygen, iron oxides are formed on the surface of the slabs, which in addition to iron (III) oxide contain a high proportion of magnetite.
properties
Black, temperature-resistant, ferrimagnetic substance with spinel structure . Insoluble in water, acids and alkalis, but soluble in hydrofluoric acid.
use
As a pigment iron oxide black for temperature-resistant, black coloring. As a magnetic pigment ( magnetic pigment ) for audio and video tapes.
One of the most important applications is as an inexpensive catalyst with a long service life in the Haber-Bosch process for the synthesis of ammonia . Magnetite also serves as a catalyst component in the dehydrogenation of ethylbenzene to styrene .
Individual evidence
- ↑ Entry on iron oxides. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.
- ↑ a b c d e Entry on iron (II, III) oxide in the GESTIS substance database of the IFA , accessed on December 21, 2019(JavaScript required) .
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1647.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .




