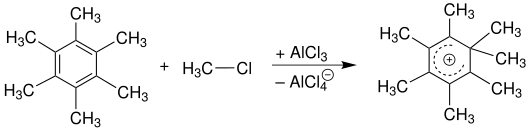Friedel-Crafts alkylation
The Friedel-Crafts alkylation is a name reaction in organic chemistry and named after its discoverers Charles Friedel (1832–1899) and James Mason Crafts (1839–1917). It is an electrophilic aromatic substitution (short: S E Ar). Under the catalytic effect of a Lewis acid (e.g. FeCl 3 , AlCl 3 , H 2 SO 4 , H 3 PO 4 , HF, HgSO 4 ), an aromatic with an alkyl halide , alcohol , alkene or alkyne becomes in Friedel-Crafts alkylation implemented. This creates alkyl aromatics.
The alkylation can also take place intramolecularly. One example is the synthesis of tetralin.
Reaction mechanism
The mechanism is illustrated in the following section using the reaction of an alkyl chloride with benzene. The Friedel-Crafts alkylation is initiated by the Lewis acid coordinating to the chlorine of the alkyl halide 1 and thereby further positiveizing the neighboring carbon ( 2 ). The complex thus formed can act as an electrophilic reagent. In the event that the alkyl radical is able to form a stable carbenium ion 3 (e.g. tert- butyl cation), it can also attack the aromatic (here: benzene) electrophilically. This temporarily removes the aromaticity of the ring. After the release of a proton, the preliminary end product 5 rearomatizes .
Alkenes as an alkylating reagent and other variants
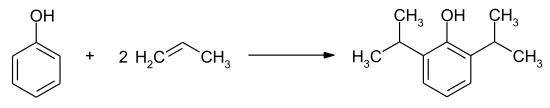
Alcohols or alkenes are also used as alternative alkylating reagents ( starting materials , starting materials) in Friedel-Crafts alkylation . However, these must first be activated by catalysts . In addition to aluminum chloride, iron (III) chloride and bromide, phosphoric acid, sulfuric acid, hydrogen fluoride or mercury (II) sulfate can also be used as a catalyst. The preparation of Propofol is effected by a Friedel-Crafts alkylation of phenol with propene :
The synthesis of butylhydroxytoluene , a versatile and technically important compound, also follows this mechanism.
Multiple substitution
An already existing alkyl group on the aromatic activates the ring against further substitution reactions. The reason for this is their positive inductive effect (+ I). The consequence of this is that poly-substituted products can often be isolated in the case of alkylations. Polyalkyl aromatics can be obtained by using an excess of alkylating reagent. In the case of benzene , it should be noted that the introduction of an alkyl radical into an unsubstituted ring loses the equivalence of the positions on the ring. Substituents have a so-called directing influence on the position of the subsequent substitution reaction. One speaks of ortho , meta and para directing substituents ( see: constitution , constitutional isomerism ). The multiple substitution is usually undesirable since, due to the low inductive effect of the alkyl group, a product mixture of several isomers is often obtained. Because of the increased affinity of the aromatic for electrophiles with each newly added alkyl group , it is possible, for example, to introduce a seventh methyl group in hexamethylbenzene . The reaction then ends with a relatively stable phenonium ion .
In order to obtain monosubstituted products, one can work with an excess of the aromatic, among other things. The overreaction does not take place in this case because, for statistical reasons, only the excess starting material and not the monosubstituted product is attacked. Multiple substitution can also be prevented if the primary product cannot accept any further alkyl groups for steric reasons, and finally, intramolecular Friedel-Crafts alkylations also offer the possibility of selective monoalkylation. Reactions of this type are ring closure reactions. The few possibilities for selective monoalkylation are the reason why Friedel-Crafts alkylation is not suitable for expensive benzene derivatives.
Rearrangement problem
Problems arise particularly in the conversion of primary alkyl halides and alcohols . The primary carbenium ion produced by the action of the catalyst tends to stabilize itself through rearrangement . Usually the Wagner-Meerwein rearrangement occurs here . The resulting isomeric carbenium ion can now also serve as an electrophile, which leads to the formation of undesired by-products.
Transalkylation
Friedel-Crafts alkylation is a reversible reaction . Therefore, alkyl residues of alkyl aromatics can be transferred to other aromatics by heating and under the influence of a catalyst.
Kinetic and thermodynamic control
Under relatively mild reaction conditions, alkyl groups are ortho- and para -directing. The result is the kinetically controlled end product. At higher temperatures or with strong Lewis acids , the thermodynamically more stable meta products are preferably formed .
See also
literature
- Magnus Rueping, Boris J Nachtsheim: A review of new developments in the Friedel – Crafts alkylation - From green chemistry to asymmetric catalysis . In: Beilstein Journal of Organic Chemistry . tape 6 , January 20, 2010, doi : 10.3762 / bjoc.6.6 .
Web links
Individual evidence
- ^ Entry on Friedel-Crafts reaction. In: Römpp Online . Georg Thieme Verlag, accessed on 2013-10-18.
- ^ GA Olah: Friedel-Crafts and Related Reactions . Wiley, New York 1963-1964, Volumes 1 and 2.
- ^ T. Laue, A. Plagens: Name and keyword reactions of organic chemistry . 5th edition, Teubner Studienbücher Chemie, 2006, ISBN 3-8351-0091-2 , p. 133.
- ^ A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag, Stuttgart 2001, ISBN 1-58890-031-2 .
- ^ AJ Kolka, JP Napolitano, GG Ecke: The ortho-Alkylation of Phenols. In: J. Org. Chem. 21, 1956, pp. 712-713, doi: 10.1021 / jo01112a621 .