Propofol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Propofol | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 12 H 18 O | ||||||||||||||||||
| Brief description |
colorless to very light yellow, clear liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 178.27 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.96 g cm −3 |
||||||||||||||||||
| Melting point |
18 ° C |
||||||||||||||||||
| boiling point |
256 ° C |
||||||||||||||||||
| Vapor pressure |
0.4 Pa (25 ° C) |
||||||||||||||||||
| pK s value |
11.1 (20 ° C) |
||||||||||||||||||
| solubility |
Water: 124 mg l −1 (25 ° C) |
||||||||||||||||||
| Refractive index |
1.5140 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Propofol is a drug from the group of narcotics which is considered to be easily controllable due to its rapid onset of action and its short plasma half-life and relatively low accumulation . In commercially available preparations, propofol is dissolved in a milky-white emulsion . It acts as an allosteric modulator on pentameric ion channels such as GABA A receptors and nicotinic acetylcholine receptors .
It was added to the WHO Essential Medicines series in 2016. John B. Glen received the Lasker ~ DeBakey Clinical Medical Research Award for the development in 2018 .
Presentation and extraction
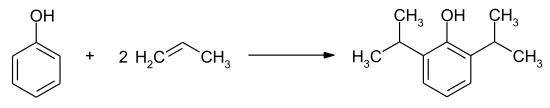
Propofol is produced by Friedel-Crafts alkylation of phenol with propene .
However, the reaction does not proceed as clearly as stated, and a large number of by-products with often very similar physical properties make it difficult to prepare the active substance in pure form. The extensive patent literature describes various process variants, including a. with the reactants 4-hydroxybenzoic acid and isopropanol , from which the intermediate 4-hydroxy-3,5-diisopropylbenzoic acid is formed in 75% yield with concentrated sulfuric acid in a Friedel-Crafts alkylation . In a second step, the benzoic acid derivative is decarboxylated to 2,6-diisopropylphenol (93.5% yield) .
After multiple extractions and vacuum distillation, propofol is obtained in a purity of 99.93%.
Clinical information
Propofol acts as a hypnotic , but has no analgesic effects. It is used for sedation , for example during gastrointestinal or colonoscopy , during somnoendoscopy or together with an analgesic to induce and maintain general anesthesia . Particularly positive characteristics are the relatively comfortable falling asleep and waking up, as well as the fact that nausea and vomiting ( postoperative nausea and vomiting ) occur less frequently than with anesthetic gases .
type of application
To induce and maintain intravenous anesthesia, propofol is used together with a short-acting analgesic - e.g. B. Remifentanil - used. It is administered using a syringe pump . The aim is to build up and maintain a sufficient blood concentration. For this purpose, a bolus dose of about 1-3 mg / kg body weight is initially administered, followed by a continuous infusion of the order of about 3-12 mg / kg / h. The injection pain caused by propofol can be reduced by giving lidocaine intravenously , either mixed with propofol or injected before the propofol injection . With computer-controlled syringe pumps that are programmed with pharmacokinetic data from Propofol, the infusion rate can be designed so that the blood level of the active ingredient remains constant or that the anesthetist can change the dosage quickly (TCI = “ target controlled infusion ”).
In the intensive care Propofol is also used for sedation applied. Since it has no pain-eliminating effect, it is usually combined with an opioid (such as sufentanil , piritramide ). After a longer infusion, degradation products can cause the urine to turn brown to green . Since it is mixed with soybean oil in the commercially available preparations, the fat metabolism is checked by the anesthetist.
Propofol can be used in patients who have risk factors for malignant hyperthermia , a rare anesthesia-related complication (myopathies, previous malignant hyperthermia, hereditary predisposition). This also applies to the treatment of overt malignant hyperthermia, since propofol, in contrast to the frequently used inhalation anesthetics, is not a trigger substance for this disease.
Pediatric anesthesia can be performed from the age of one month, whereby the use of the two percent propofol concentration is reserved for children from three years of age due to the difficult titration. Pediatric anesthesia with propofol as a hypnotic are often performed today.
Contraindications
Propofol should not be given in patients with circulatory insufficiency or hypovolaemia , as this can lead to an increased drop in blood pressure. Furthermore, it is not indicated during pregnancy and breastfeeding , as there is insufficient data on possible damage. For sedation of children under 16 in intensive care Propofol should not be used as safety and efficacy have not been established. In their technical information, the manufacturers state a soy allergy as a contraindication for the use of propofol, because propofol is dissolved with soy lecithin and an allergic reaction is conceivable. In current scientific publications, however, the use of propofol in soy allergy is considered uncritical.
Adverse Effects and Abuse
Significant side effects when administering propofol are respiratory depression up to apnea as well as a drop in blood pressure , on the one hand due to reduced peripheral vascular resistance, on the other hand due to a reduction in cardiac output (cardiodepressivity). This applies in particular to elderly patients with previous cardiac damage and thus to a large proportion of people who have to undergo general anesthesia. This limits the sensible use of propofol for induction of general anesthesia or implementation of total intravenous anesthesia (TIVA) in these patients. Arousal phenomena (spontaneous movements, muscle spasms ), allergic reactions ( anaphylaxis ) due to the release of histamine, and dreams (mostly pleasant, but also now and then bad dreams that are actually experienced, so-called bad trips ) also occur. Dreams with sexual fantasies occasionally lead to accusations of sexual harassment or even sexual abuse by the doctor. Another undesirable effect is the local pain during the injection , which can be caused by irritation of the vein wall. In addition, seizures may occur in individual cases after using propofol.
Due to the dosage form as an oil-in-water emulsion of propofol, which is practically insoluble in water, the growth of microorganisms is promoted; if handled improperly, microbial contamination can lead to serious septic complications.
In rare cases, prolonged use can lead to severe metabolic imbalances with lactic acidosis , cardiovascular failure, muscle breakdown ( rhabdomyolysis ) and acute kidney failure , the propofol infusion syndrome (PRIS), which is associated with high mortality.
Propofol has a short-lasting euphoric effect and has a primarily psychological dependency potential . Cases of abuse and dependence occur predominantly in people to whom the substance is accessible through their occupational activity and often end fatally because of the narrow therapeutic range , the omnipresent danger of respiratory depression, but also because of the lack of antagonists. Propofol is currently (as of January 2020) not subject to the Narcotics Act in Germany.
Analytics
The reliable qualitative and quantitative determination of propofol and its metabolites in urine samples is possible after adequate sample preparation through the coupling of chromatographic methods such as B. gas chromatography or HPLC with mass spectrometry as trimethysilyl derivatives . Even more sensitive detection in the picogram range is achieved through derivatisation with diazonium salts .
Pharmaceutical information
The phenol derivative propofol is hardly soluble in water and, as a result, cannot be formulated as a purely aqueous injection solution. Because of its lipophilicity , propofol is therefore dissolved in an oil, for example soybean oil , which is then emulsified with egg lecithin to form droplets that are finely distributed in an aqueous phase (emulsion). After approval in Germany in 1988, the propofol emulsion was introduced to the market by ICI (now AstraZeneca ) under the name Disoprivan . In 1989 it was approved by the Food and Drug Administration in the USA . A further development are emulsions using a mixture of triglycerides of medium- and long-chain fatty acids (MCT / LCT), which reduce the injection pain when propofol is administered and are intended to reduce the burden on lipid metabolism and are also suitable for patients with soy hypersensitivity.
Propofol is also used in veterinary medicine (dog, cat, rabbits, amphibians, to cattle and horses) as an anesthetic or for introducing (intubation) anesthesia use.
A prodrug of propofol is fospropofol , which has a less rapid rise in plasma; however, because of its water solubility, it does without lipids and emulsifiers.
Mode of action
GABA A receptors in the brain , where propofol intensifies the effect of the transmitter GABA, can be regarded as a pharmacodynamically important target . It binds to β 3 subunits within the transmembrane section in the transition area to the extracellular section and near the interface to the neighboring subunit of this pentameric receptor and binds to both α 1 β 3 and homo-β 3 receptors. In higher concentrations it inhibits nicotine receptors . A non-specific effect on lipid membranes and changes in protein subunits of sodium channels were also discussed .
Others
In the absence of any other active ingredient, propofol is to be used in the future for executions of prisoners sentenced to death in the United States . However, the German manufacturer Fresenius Kabi tries to prevent this with trade restrictions in the USA.
Propofol also played a role in the death of American singer Michael Jackson and the conviction of his personal doctor for negligent homicide. The cause of death was acute poisoning by propofol.
There has been a supply bottleneck for Propofol since November 2019. The Professional Association of German Anesthetists ( BDA ) sees this as a threat to the high level of treatment in Germany. As a result of the drastic increase in demand over the past few weeks due to the Covid-19 pandemic , the manufacturer reported a supply bottleneck for propofol and isoflurane at the Federal Institute for Drugs and Medical Devices in 2020 . The authority sees "partially restricted availability" and delivery delays, but no delivery interruptions.
Trade names
Disoprivan (D, CH), Diprivan (A, E), Recofol and generics, Cryotol (Mexico), Anespro (Venezuela), Ansiven (CH), Bioprofol (Brazil), Gobbifol (Argentina), Pantoprofol (Africa)
Individual evidence
- ↑ European Pharmacopoeia Commission (Ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0-5.8 , 2006.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X .
- ↑ a b c d e f Entry on propofol in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-442.
- ↑ a b c data sheet 2,6-diisopropylphenol from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ^ A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag, Stuttgart 2001, ISBN 1-58890-031-2 .
- ^ AJ Kolka, JP Napolitano, GG Ecke: The ortho-Alkylation of Phenols. In: J. Org. Chem. 21, 1956, pp. 712-713, doi: 10.1021 / jo01112a621 .
- ↑ Patent EP2516369B1 : Process for the production of extra-pure 2,6-diisopropylphenol. Registered on August 10, 2010 , published on July 22, 2015 , applicant: Harman Finochem Ltd., inventor: KP Jain, DU Edaki, HS Minhas, GS Minhas.
- ↑ U. Jost, C. Dörsing, C. Jahr, M. Hirschauer: Propofol and postoperative nausea and / or vomiting. In: Anaesthesiologist. 46 (9), September 1997, pp. 776-782. PMID 9412258 .
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York a. a. 1999, ISBN 3-540-65024-5 , p. 21 f.
- ↑ Lidocaine to reduce the pain caused by propofol during induction of anesthesia in adults. In: Cochraine.org. February 18, 2016, accessed June 12, 2020 .
- ^ Specialist information of the Swiss Medicines Compendium : Disoprivan 1% / 2%, Astrazeneca.
- ^ Philipp Niebel, Hinnerk Wulf: Declaration of Helsinki on patient safety in anesthesiology - Part 4: SOP on perioperative anaphylaxis. In: AINS - Anästhesiol Intensivmed Emergency Med Schmerzther. 48, 2013, pp. 230-232, doi: 10.1055 / s-0033-1343755 .
- ↑ Pascale Dewachter, Claudie Mouton-Faivre, Mariana C Castells, David L Hepner: Anesthesia in the patient with multiple drug allergies: are all allergies the same? In: Current Opinion in Anesthesiology. 24, 2011, pp. 320-325, doi: 10.1097 / ACO.0b013e3283466c13 .
- ↑ B. Balasubramaniam, GR Park: Sexual hallucinations during and after sedation and anesthesia. In: Anaesthesia . 58 (6), Jun 2003, pp. 549-553. PMID 12846619 .
- ^ V. Marchaisseau, A. Molia, E. Herlem, ML Germain, T. Trenque: Propofol-induced hallucinations and dreams. In: Therapy. 63 (2), March-April 2008, pp. 141-144. PMID 18561889 .
- ↑ S. Almer, M. Warntjen: Public prosecutor's investigations after propofol anesthesia: Unwanted 'side effects'. In: Deutsches Ärzteblatt. 106 (41), October 9, 2009, p. A-2031.
- ↑ German Medical Association: Communications: Serious adverse drug effects after Propofol infusions for sedation. In: Deutsches Ärzteblatt. 101 (50), December 10, 2004, pp. A-3447 / B-2911 / C-2759.
- ↑ Drug Commission of the German Medical Association: Septic Complications from Contaminated Propofol (ADR - Learning from Mistakes)
- ↑ F. Wappler: The Propofol Infusion Syndrome. Clinic, pathophysiology and therapy of a rare complication. In: Deutsches Ärzteblatt. 11, 2006, pp. 705-710.
- ↑ Udo Bonnet : Addiction and Propofol: Insatiable desire for an anesthetic. (PDF; 339 kB) In: InFo Neurology & Psychiatry. Vol. 13, No. 4, 2011. P. 40 ff. , Accessed on September 30, 2011 .
- ↑ Udo Bonnet: Assessment of the risk of addiction to propofol. In: Advances in Neurology Psychiatry. 79 (8), 2011, pp. 442-452, doi: 10.1055 / s-0031-1273411 .
- ↑ Maier, C., Iwunna, J., Tsokos, M. et al .: Deaths from propofol abuse. Survey in forensic medicine institutes in Germany, Austria and Switzerland. Anaesthesist (2017) 66: 109, doi: 10.1007 / s00101-016-0260-6 .
- ^ SY Lee, NH Park, EK Jeong, JW Wi, CJ Kim, JY Kim, MK In, J. Hong: Comparison of GC / MS and LC / MS methods for the analysis of propofol and its metabolites in urine. In: J Chromatogr B Analyt Technol Biomed Life Sci. 900, 1 Jul 2012, pp. 1-10, PMID 22672847 .
- ^ F. Vaiano, F. Mari, FP Busardò, E. Bertol: Enhancing the sensitivity of the LC-MS / MS detection of propofol in urine and blood by azo-coupling derivatization. In: Anal Bioanal Chem. 406 (15), Jun 2014, pp. 3579-3587, PMID 24414741 .
- ^ E. Burgis: Intensive course in general and special pharmacology . Elsevier, Urban & Fischer Verlag, 2008, p. 307 ( limited preview in Google book search).
- ^ A. Epstein, R. White, IH Horowtiz, PH Kass, R. Ofri: Effects of propofol as an anesthetic agent in adult lions (Panthera leo): a comparison with two established protocols . In: Research in Veterinary Science . tape 72 , no. 2 , April 2002, p. 137-140 , doi : 10.1053 / rvsc.2001.0535 , PMID 12027594 .
- ↑ Entry on fospropofol. In: Römpp Online . Georg Thieme Verlag, accessed on July 25, 2019.
- ↑ GM Yip, ZW Chen, CJ Edge et al. a .: A propofol binding site on mammalian GABAA receptors identified by photolabeling . In: Nat. Chem. Biol . tape 9 , no. 11 , 2013, p. 715-20 , doi : 10.1038 / nchembio.1340 , PMID 24056400 .
- ↑ SS Jayakar, WP Dailey, RG Eckenhoff, JB Cohen: Identification of propofol binding sites in a nicotinic acetylcholine receptor with a photoreactive propofol analog . In: J. Biol. Chem . tape 288 , no. 9 , 2013, p. 6178-89 , doi : 10.1074 / jbc.M112.435909 , PMID 23300078 .
- ^ Franz-Josef Kretz, Jürgen Schäffer: Anesthesia, intensive care medicine, emergency medicine, pain therapy. 5th edition. Springer, Berlin 2008, ISBN 978-3-540-75572-2 .
- ↑ Nicola Kuhrt: Death penalty in the USA: German company supplies active ingredient for lethal injection. In: Spiegel Online. June 15, 2012. Accessed June 16, 2012.
- ^ H. Haarhoff, R. Reichstein: Fresenius restricts propofol sales: "Death cocktails" are becoming scarce. In: taz.de. September 11, 2012, accessed September 11, 2012 .
- ↑ Michael Jackson's death was a homicide . ( archive.org [accessed June 13, 2020]).
- ↑ Sebastian Fiebiger: The anesthetic propofol is running out. In: Medicine +. Retrieved November 27, 2019 .
- ↑ Martin U. Müller: Strong demand for supply bottlenecks for anesthetic drugs. In: spiegel.de. April 10, 2020, accessed April 13, 2020 .