Lecithins
Lecithins (or lecithins ; from Greek λέκιθος lekithos , German 'egg yolk' ) is the classic name for a group of chemical compounds, the so-called phosphatidylcholines . These are phospholipids , which are made up of fatty acids , glycerine , phosphoric acid and choline . Lecithins are components of the cell membrane of animal and plant life. However, they do not occur in the membranes of most bacteria. They are accompanying substances in fats and oils and particularly rich in eggsyolks and cells of vegetable seeds present.
Lecithins allow the emulsification (mixing) of fats and water and are therefore important natural surfactants ( emulsifiers ) for food and feed. Lecithins are approved in the EU as a food additive (E 322) for food (also for " organic products") with maximum quantity restrictions only for baby food . They are listed as lecithin , soy lecithin or E 322 on ingredient lists. In medicine and cosmetics, they are also used as active ingredients, in dietetics as food supplements .
Technically obtained lecithin products such as extracts from soybeans or eggs, depending on their sources, contain not only lecithins but also other phospholipids as well as sphingomyelins and glycolipids . These groups of substances also have similar physical properties and are emulsifiers. According to an EU directive, the proportion of polar lipids (insoluble in acetone ) in lecithin products must be at least 60%.
Discovery and Exploration
In 1811 the French pharmacist Louis-Nicolas Vauquelin reported for the first time about fat-containing preparations made from brain matter that contained organically bound phosphorus and that were found by the German chemist Johann Thomas Hensing as early as 1719 .
The French Nicolas-Theodore Gobley isolated a sticky, orange-colored substance from the egg yolk in 1846/1847, in which oleic acid , margaric acid , glycerol phosphoric acid and a nitrogenous organic base were present. He found comparable substances in brain matter, carp eggs, blood, bile and other organs in 1847–1858. In 1850 he named his discovery lecithin after the Greek word lekithos 'egg yolk'.
The German Felix Hoppe-Seyler , founder of biochemistry and molecular biology, found organically bound phosphorus in plant seeds in 1867 . In 1899, the German chemists Ernst Schulze and Ernst Steiger isolated phospholipids from plant seeds , which they also called lecithin. According to their findings, soybeans and lupins had the highest lecithin content of 1.5–2.5% of the plant seeds they examined.
The researchers Diakonow and Adolph Strecker (1822–1871) isolated lecithin, e.g. B. from egg yolk, in greater purity and recognized that the nitrogen-containing part of the lecithin was choline .
Johannes Ludwig Wilhelm Thudichum (1829–1901), the founder of brain chemistry, found an analogous connection and named it cephalin after the Greek word kephalos 'head' and was able to separate the sphingomyelin.
From the beginning of 1900 to the late 1930s, no significant advances in knowledge of phospholipids can be seen. Ernst Klenk (1896–1971) and Sakat found inositol and inositolphosphoric acid in soy lecithin in 1939 . In 1944, the American chemist Jon Pangborn extracted cardiolipin from the lipid of the bovine heart muscle, and in 1958 Carter and his colleagues described the complex phytoglycolipids that only occur in plant-based phospholipid mixtures .
When Hansamühle Hamburg , today ADM Ölmühle Hamburg AG, introduced the Bollmann extraction process in 1925 , lecithin could be economically isolated from raw vegetable oil. Industrial production began. The main source of lecithin was soybean oil. Lecithin from egg yolk has in special applications, e.g. B. in pharmacy and cosmetics, continues to be important.
One of the first application researchers for lecithin was Bruno Rewald around 1925 , who was one of the first lecithin technologists to recommend lecithin as an emulsifier and dispersant .
Hamburg became the starting point and center for industrial soybean and lecithin processing. The American Josef Eichberg was the first to recognize the value of lecithins for the USA in 1930 and to market Hansamühle's 'Hamburger Lecithin' there. From 1935 lecithin was also produced in America in good quality. It was the companies Pillsbury and Central Soya (both USA) who took on this versatile substance.
From 1948, Lucas Meyer , Hamburg, dedicated himself to application technology and the sale of lecithins. With Rüdiger Ziegelitz and Volkmar Wywiol , who promoted the marketing and further development of lecithin from 1953, the global breakthrough for lecithin as an auxiliary and active ingredient was achieved. They put the variety of uses of lecithins in the food, feed and technology sectors on a broad basis.
The doctor Buer did pioneering work in dietetic use and launched one of the first lecithin preparations on the market in 1935 with the product 'Buer lecithin'. H. Eickermann, A. Nattermann & Cie. (today the Sanofi-Aventis Group), concentrated on the active substance phosphatidylcholine and developed a number of important pharmaceutical preparations that are still offered today.
Herbert Rebmann developed phospholipid specialties from the egg yolk as high-quality pharmaceutical emulsifiers for fat nutrient solutions.
Research and application technology are far from over. For example, lecithins from seaweed, the use of liposomes in the food industry and phospholipids in aquaculture are currently the focus of science.
Occurrence and availability
Occurrence
Polar lipids, especially phospholipids, are important structural components of biological membranes and occur in all living beings (humans, animals, plants and algae) and in many microorganisms. The highest concentrations of lecithin are found in the liver and brain, in the lungs and heart, and in muscle tissue. Phospholipids are also found in some body fluids - especially in the blood plasma of vertebrates. Phosphatidylcholine is - as phosphatidylethanolamine - in Kennedy produced -Stoffwechselweg.
Availability
Currently around 180,000 tons of lecithin are produced annually, mainly from soybeans (2% lecithin content), which are harvested in the USA, Brazil and Argentina. Other soy producers, such as China, India, Paraguay and Canada, are currently of little importance for global lecithin production. The cultivation of soy in Europe is marginal. More than 70% of the world's soy harvest comes from genetically modified soy plants (as of 2011). In addition to soy, rapeseed and sunflowers count as raw material sources, albeit to a lesser extent. Egg yolk, with its high proportion of lecithin (approx. 10%), can hardly supply the market due to its limited availability. The relatively low amounts are mainly used in pharmaceuticals, medicine and cosmetics.
Effects of lecithin in the body
In addition to their structure-forming properties, lecithins are assigned numerous functional tasks. They are actively involved in both anabolic lipid metabolism (synthesis and distribution of lipids) and catabolic lipid metabolism (breakdown and conversion of lipids).
- The cell membrane of almost all cells consists of a lipid bilayer. Lecithin is essential for the formation of the biomembrane and parts of the cell organelles. The mitochondria in particular are dependent on components of the lecithin for their synthesis capacity with the glycoproteins associated in the molecular structure.
- Since fats are not water-soluble, various body-specific steps are necessary for the digestion of fat in order to be able to carry out the digestion that begins with the breakdown of fat droplets ( micelles ).
- The export of fatty acids from the liver is particularly important for farm animals.
Chickens primarily ingest starch with their diet , from which the liver has to synthesize fats for egg formation; Lecithin is necessary here to export the formed fats from the liver ( Very Low Density Lipoproteins , VLDL), otherwise there is a risk that the animal will develop fatty liver disease. In cows this danger also exists in part, but this is the result of a different process: shortly after the birth of the calf, the very energy-intensive milk production begins. For this purpose, body fat reserves are mobilized, which are first transported to the liver and from here in turn as VLDL into the blood. If the cows are not adequately supplied with amino acids at this point in time (in particular: lysine and methionine ), fat can also be stored in the liver, which can ultimately lead to depression in performance. Research in this area is ongoing.
Chemical structure and properties
and unsaturated oleic acid
(POPC = P almityl o leyl p hosphatidyl c holin)
Lecithins (phosphatidylcholines) are a widespread group of compounds that belong to the superordinate group of phosphoglycerides . Phosphoglycerides are compounds that combine with glycerine and two fatty acids to form a dicarboxylic acid ester . This part of the phosphoglycerides corresponds to the structure of common fats . The third OH group of glycerol, however, forms a phosphoric acid diester with a phosphate ion ; on the one hand with glycerine and on the other hand with a further, unspecified functional group X. In the case of the lecithins, the group X is choline . Choline is a quaternary ammonium compound , so it has a positive charge and is a cation . The phosphate group is present as an anion over a wide pH range and therefore carries a negative charge. Thus, lecithins can be understood as zwitterions or inner salts . Lecithins do not have a characteristic melting point because the compounds have different fatty acid compositions. Unsaturated fatty acids such as oleic acid or linolenic acid are quite common in lecithins.
The structure of these compounds leads to the property of acting as a surfactant : one part of the molecule has a polar ( hydrophilic ) property , another part an apolar ( hydrophobic ) property. They are thus amphiphilic , can reduce the interfacial tension between a wide variety of substances ( phases ) and act as emulsifiers or dispersants . They therefore allow the mixing of actually immiscible liquids such as oil and water and the suspension of particles in an aqueous phase.
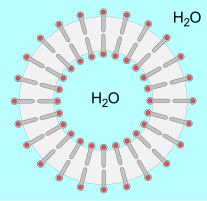
Similarly, lecithins can form liposomes that serve as models for the development of cells and can help in medicine as a transport aid for active substances. Lecithins are also able to form lamellar liquid-crystalline phases, which is of particular interest for cosmetic use.
Other phospholipids
In addition to lecithins, lecithins that are obtained from natural sources contain other phosphoglycerides such as phosphatidylethanolamine with ethanolamine , phosphatidylserine with serine and phosphatidylinositol with inositol as polar group X. There are also sphingomyelins and glycolipids , the latter not being phospholipids . These groups of compounds also show similar physical properties and act as surfactants. Natural sources of lecithins are e.g. B. Eggs and soybeans . The table shows the approximate composition of chicken egg and soy lecithin.
| Surname | polar functional group | Egg lecithin | Soy lecithin |
|---|---|---|---|
| Phosphatidylcholine |
Choline |
73 | 30th |
| Phosphatidylethanolamine |
Ethanolamine |
15th | 22nd |
| Phosphatidylserine |
Serine 
|
- | 3-4 |
| Phosphatidylinositol |
Inositol 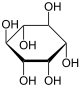
|
1 | 18th |
| Sphingocholine |
Choline |
2-3 | - |
| Glycolipids |
Monosaccharides oligosaccharides |
- | 13 |
Physical Properties
Lecithins are hygroscopic . In the air they form sticky, waxy masses. If the lecithins are heated to over 70 ° C for a long time, they turn dark brown to black. In principle, lecithins, the related phospholipids and their modified derivatives are soluble in fats and oils and dispersible in water. Lecithins are readily soluble in organic solvents such as chloroform or hexane . In contrast, they are insoluble in acetone . The solubility in ethanol depends on the chain length and the degree of saturation of the fatty acids. If the degree of saturation is low, the ethanol solubility of the phosphatidylcholine decreases. Phosphatidylethanolamine and phosphatidylinositol are poorly or insoluble in ethanol.
Lecithins should be tightly closed, protected from light and not stored above 15 ° C. Since it tends to oxidize with molecular oxygen ( auto-oxidation ), antioxidants can be added for stabilization .
Extraction of soy lecithin
Raw material: soybean
The soybeans of the main producing countries are sufficiently available as a renewable raw material (2005 harvest: 214 million t). Matured and carefully stored beans are of great importance for good lecithin qualities. The beans must first be cleaned, broken and rolled into flakes.
Raw material: raw soybean oil
The platelets (2-5 mm) are extracted in an extraction system in countercurrent with hexane. The resulting mixture ( miscella ) is distilled and evaporated and finally freed from the solvent in vacuo by adding direct steam.
The resulting crude oil is the starting product for the soy lecithin. By steaming the oilseeds before extraction , the lecithin content of the crude oil can be increased by 50–100%. The proportion of non-hydratable phospholipids in the degummed oil then falls.
Result: lecithin
The crude oil, which contains around 2% lecithin as an accompanying substance, is heated to 70–90 ° C in a source container and intensively mixed with 1–4% water. The lecithin swells, precipitates as a gelatinous mass and is separated from the crude oil with high-speed special separators. The water is removed from this lecithin wet sludge - with around 12% oil, 33% phospholipids and 55% water - in a thin-film evaporator. The result is a raw lecithin that contains 60–70% polar lipids and 27–37% soybean oil. The water content is now only 0.5–1.5%.
The main components of the raw lecithins obtained through degumming are: phospholipids (also known as phosphatides), triglycerides , glycolipids and carbohydrates . Minor ingredients: sterols , free fatty acids , coloring agents and a number of other compounds.
In addition to degumming using the swelling process with water, there is degumming with acids (super degumming) and a degumming process with the enzyme phospholipase A2. In particular, the so-called non-hydratable phospholipids, which are otherwise impossible or difficult to detect, are also precipitated.
Pure lecithin - fractions - modifications
Lecithin can be used in its original form for many applications. In many cases, however, it makes sense to de-oil, fractionate or modify native (original) lecithin in order to obtain specific lecithins for special applications:
- De-oiling is used to produce powdered or granulated “pure lecithins” by removing the oil and free fatty acids from the native lecithin. They are tasteless, easy to dose, have a high phospholipid concentration and have improved O / W emulsifying properties.
- Fractionation means the separation of the lecithin complex into an alcohol-soluble and an alcohol-insoluble component. The alcohol-soluble fraction can be further divided into two fractions with specific properties using a chromatographic process.
- The modification is based on the separation of a fatty acid molecule from the phospholipid molecule. This is done with the help of phospholipase A2 . The process is called enzymatic hydrolysis. The resulting "lysolecithin" is particularly hydrophilic, which increases the O / W emulsifying properties and increases calcium ion tolerance.
- Acetylation, another form of modification, changes the phosphatidylethanolamine by adding an acetic acid molecule to the amino group. This makes the resulting lysolecithin particularly hydrophilic.
- Another possibility for improving the emulsifying activity of the lecithins is the hydroxylation of the mono- and polyunsaturated fatty acids bound in the phospholipid molecule. This is done by reacting with hydrogen peroxide.
properties
Raw vegetable lecithins are brown to yellowish substances with a plastic and liquid consistency . The color depends on the origin of the seeds, the harvest and storage conditions and the processing methods and equipment. The consistency is determined by the oil content, the amount of free fatty acids and the moisture content. De-oiled lecithins are powdery or granulated. Well-cleaned (refined) lecithins have a characteristic (beany) to neutral smell and taste. In principle, lecithins, their modified derivatives and the fractionated phospholipids are soluble in oils and fats.
Uses of lecithin
The use of lecithin in food and feed production, in pharmacy and medicine as well as in cosmetic products and in the non-food area is diverse. Some possible uses are shown below.
Lecithin in foods
The largest amount of industrially produced lecithin, mainly from soybeans, goes to the food industry. Initially, vegetable lecithin was a substitute for egg lecithin. But it has long been considered to be of equal value, sometimes even to be superior. It has a permanent place as an emulsifier and dispersant , both for hydrophilic substances in oily and hydrophobic in aqueous media and as a stabilizer of interfaces in gaseous / aqueous and gaseous / solid food systems. Soy lecithin from genetically modified plants is usually undetectable as it no longer contains any DNA from the plant due to the manufacturing process. Lecithin from genetically modified plants is subject to labeling.
- Lecithin in bread and baked goods : Lecithins are primarily important auxiliary substances in baking processes . They make it easier to whip fatty doughs and allow the use of low-gluten doughs. The higher volume yield, finer pores and crispy crust that can be achieved are particularly beneficial for bread roll production. The ability of lecithin to retard the staling of bread and baked goods is particularly important. 0.1-0.3% pure lecithin is added to the dough. Commercial lecithin powder consists of 50–95% flour, this dilution makes it easier to dose small amounts.
- Lecithin in the production of margarine : In the beginning, margarine had the disadvantage compared to butter of splashing when frying and the firm adhesion of milk casein , which burned with an unpleasant odor. To prevent this, lecithin from egg yolk was used as an emulsifier . However, the problems could only be solved economically with tasteless soy lecithin. New process technologies and recipes ensured a significant increase in quality, which however led to the abandonment of native soy lecithin and towards special lecithin fractions. This also made it possible to achieve better oxidation resistance and stabilization. A good anti-splash effect with half-fat margarine (40% fat and 60% water) cannot be achieved with the addition of lecithin alone. This is only possible in connection with surface-active substances such as soy protein concentrates .
- Lecithin in chocolate : As almost everywhere, lecithin also has a double function here: an increase in the quality of the chocolate and a number of advantages in production. In order to achieve the right consistency and the typical aroma in chocolate production , the mass has to be stirred in the conche for several hours . The use of lecithin reduces the viscosity , shortens the processing time and saves cocoa butter . But the properties are also favorably influenced. The chocolate becomes more resistant to elevated temperatures, the shelf life is extended, the gloss of the surface is increased and premature graying is reduced. The industry almost exclusively uses soy lecithins, but lecithins from rapeseed and sunflower seeds are also used. Synthetic lecithins and lecithin combinations can prove beneficial in production. The same applies to the use of lecithin fractions, which allow the chocolate to liquefy better during conching than native lecithins.
- Lecithin in instant foods: Plant lecithins have proven themselves in instantizing cocoa and coffee powder. But they can be used particularly effectively with whole and skimmed milk powder. They also serve as dispersants in soy protein products, potato starch and dry soups.
- Lecithin in ice cream : Auxiliary substances such as binders, emulsifiers, stabilizers, vegetable fats as well as flavorings and colorings are often added to industrially produced ice cream. Lecitin found in egg yolks is often used as a natural emulsifier. With its help, a very fine distribution of the fat droplets in the water is achieved and creaming, i.e. the separation of the fat from the water, is prevented. Frozen desserts such as ice cream or sorbets in particular contain a large amount of emulsifiers.
- Lecithin is 0.3% used in chewing gum .
Lecithin in medicine
For the treatment of ulcerative colitis , preparations containing enteric coated lecithin are available on the market. Lecithin is present in the colon mucous membrane in humans and protects it from food components and intestinal bacteria. In ulcerative colitis patients, the amount of lecithin is reduced. The drug delivery of the lecithin is intended to counteract this deficiency on the spot. It is necessary to protect the lecithin preparation by means of enteric capsules or microencapsulation because pancreatic enzymes are able to break down the lecithin. Accordingly, unprotected lecithin preparations available as food supplements are already broken down in the area of the duodenum and are therefore unsuitable for ulcerative colitis patients.
Lecithin in animal feed
- Calves and cattle : In feed for cattle, lecithin is primarily of technological importance. Lecithin prevents z. B. dust formation in the production of farinaceous concentrate mixes. At the same time, the risk of dust explosions during production isreduced. Due to its emulsifying properties, it delays the creaming of the fat and the sedimentation of insoluble components in the drink. In the young animals, however, the physiological aspects are more in the foreground. With calf milk replacers, in which the cow's milk is replaced with skimmed milk and enriched with non-dairy fats and proteins, very good feeding results - at least comparable to cow's milk - are achieved.
- Pigs : The use of lecithin in artificial sows' milk, which is often necessary in piglet rearing, is about the same as in calf milk replacers. The addition of lecithin in the fattening feed causes a significantly better fat utilization, so that the fattening time can usually be reduced.
- Chickens : Faster growth and increased vitamin A storage in the liver could be demonstrated in chicks when they ingested lecithin with the feed. The fatty liver syndrome, which has been described in Germany since 1956, ispositively influencedby the addition of lecithin - with its high proportion of choline and inositol - to laying hen feed. The psoriasis also stopped . The laying performance and egg weight of hybrids can also be increased.
- Aquaculture : In trout and salmonid breeding, the indigestibility of fats leads to problems and an increased death rate. Sea animal oils also caused liver and kidney damage and depigmentation , so that trout were fed fat-free for a long time. Lecithins and poultry fat, however, have a beneficial effect on the health and growth of the fish. The linoleic and linolenic acid and the choline of the lecithin promote growth and increase feed conversion. Kidney and intestinal bleeding as well as fatty liver syndrome can be avoided. The use of lecithin in aquaculture is becoming increasingly important, for example in the breeding of crustaceans , shellfish and seafood .
- Fur animals : At first it was only assumed that the feeding of lecithin, e.g. B. in rabbits and mink, could be useful to prevent liver diseases - especially during pregnancy. Later feeding attempts proved it. The growth is faster, the pelts of the young mink are bigger and of better quality. Fatty liver is widespread in mink. This is due on the one hand to a mostly one-sided diet, but also to missing substances such as those in lecithin and which have proven themselves in liver metabolism.
Lecithin in the non-food sector
Today, in a wide variety of industries, for economic reasons, aqueous emulsions made from oils, fats and waxes are used instead of pure oil. The lecithin plays an outstanding role as an emulsifier and active ingredient. About 20% of the lecithin used goes into the technical industry.
Important areas of application are the construction industry, the treatment of construction timber, the use of man-made fibers, clothing and glove leather, leather care products, the melting of wool, dyeing or printing on fabrics and in the transport of coal through pipelines and in paints and varnishes.
Lecithin in cosmetics:
Phospholipids are lipid replenishing. They prevent normal and especially dry skin from drying out after washing. This property is also used in shampoos. They regulate the pH value of the skin and support the natural protective layer against aggressive environmental influences. Their high content of linoleic and linolenic acid has a positive effect on skin diseases. The application of phospholipids for the production of spherical vesicles, the liposomes, is relatively new . The spheres are bounded by lipid double membranes made of phospholipids. Inside, they contain an aqueous phase in which special active ingredients are dissolved, which can then be more easily introduced into the skin.
Phospholipids in plant protection products :
In addition to the active ingredients, pesticides contain solvents and emulsifiers and possibly other auxiliaries. If lecithin is the emulsifier, it reduces the biocidal active ingredient content while increasing the effectiveness of the remaining active ingredient content. The aqueous emulsion remains stable and enables the active ingredients to be better distributed.
As a physically effective insecticide, lecithin is also used to combat mosquito larvae and pupae. Lecithin forms a particularly thin film on the surface of the water, which prevents the mosquitoes from adhering to the aquatic developmental stages of their respiratory organs. These die due to a lack of oxygen without harming other organisms.
Individual evidence
- ↑ Suzanne Jackowski, John E. Cronan, Jr., Charles O. Rock: Lipid metabolism in procaryotes . In: Dennis E. Vance, J. Vance (Eds.): Biochemistry of Lipids, Lipoproteins and Membranes . Elsevier, 1991, ISBN 0-444-89321-0 , pp. 80-81.
- ↑ F. Gibellini, TK Smith: The Kennedy pathway - De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. In: IUBMB life. Volume 62, Number 6, June 2010, pp. 414-428, doi: 10.1002 / iub.337 . PMID 20503434 .
- ↑ transgen.de: Food for Europe's farm animals: Hardly any alternatives to the import of (genetically modified) soybeans. ( Memento of the original from January 22, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. February 23, 2012.
- ^ Technical lexicon ABC chemistry. Vol. 2. 3rd edition. Harri Deutsch, Frankfurt 1987, p. 855, ISBN 3-87144-899-0 .
- ↑ transgen.de: Lecithin. possible application of genetic engineering. ( Memento of the original from February 27, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. February 27, 2015.
- ↑ J. Schormüller: Lexicon of food chemistry . 2013, ISBN 978-3-642-65779-5 , pp. 488 .
- ↑ dradio.de: Questionable food additives: Emulsifiers disrupt the intestinal flora. February 27, 2015.
- ^ Richard W. Hartel, Joachim H. von Elbe, Randy Hofberger: Confectionery Science and Technology . Springer, 2017, ISBN 978-3-319-61740-4 , pp. 398 .
- ↑ http://medctr.de/colitis-ulcerosa/#lecithin
- ↑ Phosphatidylcholine - mucous membrane protection as a therapeutic and prophylactic principle , on klinikum.uni-heidelberg.de, accessed on March 14, 2017
- ^ DS Kettle: Medical and Veterinary Entomology - Second Edition, (1995), Cab International
literature
- Hermann Pardun : Plant Lecithins . Extraction, properties, processing and application of plant-based phosphatide preparations. Ziolkowsky, Augsburg 1988, ISBN 3-87846-128-3 .
- Werner Schäfer, Volkmar Wywiol: Lecithin - The incomparable active ingredient . Strohte, Frankfurt 1986, ISBN 3-87795-031-0 .
- Rüdiger Ziegelitz: Lecithins - Properties and Applications . Lucas Meyer, Hamburg 1989.
- Jean Pütz, Christine Niklas: Make-up, masks, beautiful hair - gentle cosmetics . vgs Verlagsges., Cologne 1987, ISBN 3-8025-6151-1 .
- DD Lasic: Liposomes. From Physics to Applications. Elsevier, Amsterdam 1993, ISBN 0-444-89548-5 .
- Dietrich Arndt, Iduna Fichtner: Liposomes, presentation - properties - application . Akademie-Verlag, Berlin 1986, ISBN 3-05-500148-6 .
- Rüdiger Ziegelitz, Lutz Popper: Lecithin - proven functionality (PDF; 743 kB) . In: bmi aktuell. Edited by Baking agent institute . Bonn 2005.1 (May). (pdf).
- Tamas Balla: Phosphoinositides. Tiny lipids with giant impact on cell regulation . In: Physiological reviews . tape 93 , no. 3 , 2013, p. 1019-1137 (English).
Web links
- Lecithin / soy lecithin , on phytodoc.de