Fats
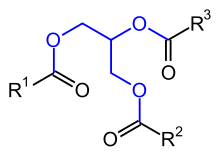
Fats and fatty oils ( neutral fats ) are esters of the trihydric alcohol glycerine (propane-1,2,3-triol) with three, mostly different, predominantly even and unbranched aliphatic monocarboxylic acids , the fatty acids . Compounds of this type are also called triglycerides , but the IUPAC recommends triacylglycerine as a name .
Depending on whether a fat is solid or liquid at room temperature , it is called fat or fatty oil . The best known fats are the eponymous mixtures of substances made from various fatty acid triglycerides obtained from animals, the term fatty oil distinguishes the (thin) liquid fats from other groups of oils (generally unspecifically diverse groups of liquid organic compounds).
As natural substances , fats are assigned to lipids and are soluble in lipophilic organic solvents such as petroleum ether , ether and benzene . With an energy density of 37 kJ / g (9 kcal / g), fats are the most important energy store for humans, animals and some plants. In plants, fats are mainly found in seeds or germs , in the animal organism in fatty tissue . Fats and fatty oils are used as food ( edible fats and oils).
etymology
The word fat is a noun of the originally low. Adjective mnd. vet ( oberd . : feist ), which in turn is the 2nd participle of the im Nhd. submerged verb mhd. veiȥen represents "make fat". It is based on an extension of the Indo-European. Root PE [i] - "are full, fat be".
Extraction
Fats are obtained either from animal products or from plants ( useful plants ), sometimes also in the chemical industry . Animal fats are either melted directly from fatty tissue ( lard , oil , sebum ) or obtained from milk ( butter ). The vegetable oils and fats used for food are obtained from oil plants or oil seeds by pressing or extraction with steam or solvents . Refining and thus the removal of unwanted ingredients makes the fats usable for humans. Margarine was originally of animal origin, but is nowadays obtained by hydrogenation ( fat hardening ) of the C = C double bond (s) in the fatty acid residues of vegetable oils (sunflower oil, rapeseed oil). Trans fatty acids can also be formed, which is undesirable.
In 2006 there were 53 companies in Germany that were involved in fat extraction and refining. With 3,445 employees, a total turnover of 131 million euros was achieved. The refining of fats is an important industry with 82.7 million euros.
In 2007, 2.4 million tons of rapeseed oil, 685,300 tons of soybean oil, 47,700 tons of sunflower oil and 1,961 tons of linseed oil were produced in Germany. In 2007, rapeseed oil (1.55 million tonnes), sunflower oil (195,000 tonnes), soybean oil (510,600 tonnes) and palm oil (504,000 tonnes) were mainly refined. Most of the products are intended for export. The production of margarine (2007: 430,000 tons) and butter (2007: 1.35 million tons) is also important.
properties

The physical properties of a fat are influenced by the chain lengths and especially by the frequency of C = C double bonds in the fatty acid residues. If a fatty acid residue contains a double bond, it is called unsaturated , and if there are several double bonds, it is called polyunsaturated. Double bonds in natural fats and oils almost exclusively have the cis configuration ; If a fatty acid contains several double bonds, these are usually separated from one another by a methylene group (–CH 2 -). The illustration on the right shows a typical example of a triglyceride molecule that is found in many vegetable fats.
Natural fats usually contain different fatty acids, are always a mixture of different clearly defined fats with a uniform molecular structure and do not have a sharp melting point but a melting range. The melting temperature increases with increasing chain length and decreasing number of double bonds between the carbon atoms of the chain. The solid products contain high proportions of long and saturated fatty acids, whereas the fatty acids in the liquid oils are predominantly mono- or polyunsaturated. Vegetable fats contain many unsaturated fatty acid residues and are therefore mostly in the form of oils. When heated, some fats already decompose below their boiling point .
Fatty acids consist of four to 26, typically twelve to 22 carbon atoms and their number is practically always an even number. These relatively long chains of fatty acids shield the oxygen atoms of the ester bond, so that fats are hydrophobic and therefore hardly soluble in water . As a result, they have no influence on the osmotic state of an aqueous phase such as cell sap , intercellular fluid , blood , lymph in animals, vacuoles and transport vessels in plants. As a depot fat, they form a suitable form of storage for energy - in humans the amount for this is 10 kg and more.
Evidence has been found that a fatty taste - in addition to the already known salty, sour, sweet, bitter and umami - could represent a further quality of the sense of taste: In mice, fatty acids such as linoleic acid contained in the food lead to an activation of taste cells and nerve cells in the taste-relevant areas of the brain.
However, fats are mostly odorless and tasteless, but act as a flavor carrier. The intense odor that occurs with rancid fat comes from short-chain, released fatty acids such as butyric acid or from keto or hydroxy fatty acids, which are toxic to the human organism.
Drying oils and fats
Drying oils should correctly be called hardening oils , as they do not dry by releasing a solvent , but mostly crosslink through oxidation . This process is also called resinification or polymerization .
The higher the proportion of unsaturated fatty acids, the higher the iodine number and the more likely oils tend to polymerize. Easily polymerizing oils such as linseed oil have an iodine number of more than 140 and are called drying or hardening oils. Semi-drying oils have an iodine number between 100 and 140; for non-drying oils it is below 100.
In connection with pigments , drying oils are used as oil paints and with the addition of resins as oil varnishes .
History of oleochemistry
Michel Eugène Chevreul did the first fundamental work to elucidate the chemical structure of fats and fatty acids around 1823. In later years, Heintz worked on palmitic and stearic acid .
Fatty acid composition of some fats and oils
Fatty acids are chemically bound in triglycerides in almost all natural (vegetable and animal) oils and fats. Contrary to popular belief, natural fats and oils do not contain any free (chemically unbound) fatty acids, but glycerol esters of the fatty acids.
| Number of carbon atoms | cis double bond to | Surname | butter | olive oil | Coconut oil | linseed oil | Sunflower oil | Palm oil |
|---|---|---|---|---|---|---|---|---|
| 4 6 8 10 |
- |
Butyric acid Caproic acid Caprylic acid Capric acid |
9% | 0% | 16% | 0% | 0% | 0% |
| 12 | - | Lauric acid | 3% | 1 % | 48% | 0% | 0% | 0% |
| 14th | - | Myristic acid | 8th % | 1 % | 16% | 0% | 0% | 1 % |
| 16 | - | Palmitic acid | 22% | 10% | 9% | 5% | 8th % | 44% |
| 18th | - | Stearic acid | 10% | 2% | 3% | 4% | 8th % | 4% |
| 18th | 9 | Oleic acid | 37% | 78% | 6% | 22% | 27% | 39% |
| 18th | 9, 12 | Linoleic acid | 10% | 9% | 2% | 17% | 57% | 11% |
| 18th | 9, 12, 15 | α-linolenic acid | 0% | 0% | 0% | 50% | 0% | 0% |
| 20th | 5, 8, 11, 14 | Arachidonic acid | 0% | 0% | 0% | 0% | 0% | 0% |
physiology

Fats and oils are basic human nutrients . Among other things, they are needed in the human body as
- Energy supplier (so-called reserve material ),
- Insulators against cold,
- Solvent for only fat-soluble substances such as some vitamins ,
- Protective padding for internal organs and the nervous system ,
- Part of the cell membrane .
Fats as energy stores
Besides carbohydrates ( sugar , starch and glycogen ), fats are the most important energy stores in cells. The physiological calorific value of 37 kJ / g fat is more than twice as high as that of carbohydrates and proteins (17 kJ / g).
The depot fat as an energy store in the human body comes from the fat contained in food or from other macronutrients (carbohydrates, proteins), which can ultimately be converted into fat over several intermediate stages if there is an excess of energy. It is controversial to what extent the conversion of the macronutrients fat, carbohydrates and proteins directly contributes to the formation of adipose tissue. Such a connection is established especially from the point of view of calorie theory . Other mammals can easily make depot fats from an excess of energy in their food.
The density of human adipose tissue is 0.94 kg / l, the physiological calorific value (energy content) is around 29,000 kJ / kg (7,000 kcal / kg). The difference to 37,000 kJ / kg of fat results from the fact that the adipose tissue does not consist of pure fat. In the blood of people who Gesamttriglyceride are determined and are, as such, in addition to the cholesterol level of the blood fats. The normal level of triglycerides in the blood is 70 to 170 mg / dl.
According to the German Nutrition Society (DGE), a fat intake of 60 to 80 g per day is sufficient for an adult , which corresponds to 25 percent of the energy consumed from food. There may well be small excesses, provided that the fat intake is balanced out in the following days. Women should consume a maximum of approx. 420 g and men approx. 560 g of fat per week. This calculation is based on an assumed energy requirement of approx. 10 to 13.4 MJ per day (= 2,400 to 3,200 kcal / d ). This would correspond, for example, to a 40-year-old office worker with a body weight between 80 kg and 107 kg who does not do regular sport. Only the determination of the actual resting metabolic rate and the individual physical activity allows an exact determination of needs. Concomitant illnesses must also be taken into account.
Trans fatty acids can stress the body and lead to vascular damage.
Biosynthesis of fats
The triacylglycerols are built up from the components glycerol and fatty acids in several reaction steps.
First, the fatty acid is activated using one of several fatty acid CoA ligases and glycerol using one of the glycerol kinases . The end products acyl-CoA and glycerine-3-phosphate react to form lysophosphatidic acid , catalyzed by the enzyme glycerine phosphate O- acyltransferase . Another fatty acid molecule is transferred by the acylglycerol-3-phosphate- O- acyltransferase , resulting in phosphatidic acid . One of the phosphatidate phosphatases splits off phosphate , leaving diacylglycerol . Finally, the diacylglycerol- O- acyltransferase transfers a third fatty acid molecule to the triacylglycerol.
Breakdown of fats
In fat cells , triacylglycerols are surrounded by a shell made from the protein complex Perilipin : CGI-58 , which, depending on the degree of phosphorylation, prevents the breakdown of fats through hydrolysis . The enzyme hormone-sensitive lipase (HSL) is responsible for starting the breakdown and is subject to both positive ( catecholamines , ACTH , glucagon ) and negative regulation ( insulin ).
The triacylglycerols are broken down in twelve steps: after HSL has been phosphorylated and dimerised, the protein layer around the lipids is broken up with catecholamines or glucagon , perilipin separates from CGI-58 and is phosphorylated by protein kinase A and later recycled with protein phosphatase 1 . HSL gets close to the lipids; their hydrolysis activity is enhanced by complexation with FAB4 . In this way, fatty acids and cholesterol are formed from cholesterol esters, and glycerol and three molecules of fatty acid are formed from triacylglycerol. Dephosphorylation of HSL terminates the process, the identity of the phosphatase that catalyzes this reaction being unknown. The course of the entire metabolic pathway was deduced from rat and mouse cells.
use
The use of fats and fatty oils (the latter are usually colloquially referred to as oils for short) as food and in food preparation and preservation is widespread. Significant amounts of vegetable oils (rapeseed oil, palm oil) have recently been chemically converted into biodiesel . For this purpose, the oils are subjected to a transesterification with methanol in the presence of acidic heterogeneous catalysts . This produces fatty acid methyl esters (FAME) and glycerine . Fatty acid methyl esters are sold directly as biodiesel, but much larger quantities are already mixed with conventional diesel fuel in the refineries of the mineral oil industry. The legislature has issued regulations for this, according to which an admixture of up to 5% by volume of fatty acid methyl esters is permitted without labeling the fuel and is also widely practiced. The fatty acid methyl ester must meet certain well-defined quality parameters in the standard DIN defined EN 14,214th
The direct combustion of melted fats and oils in truck diesel engines is common. However, the vehicles must be specially converted beforehand.
By saponification (ester splitting with alkali hydroxides), soaps , the alkali salts of fatty acids, are produced from fats or fatty oils . This also produces glycerine.
Analytics
The fat content of foods is usually determined by extraction with lipophilic solvents. The FDA defines fat as the saponifiable portion of a food. This means that non-acylglycerides, such as sterols or phosphatides, do not fall under the FDA definition of fat.
For fat characterization, titration- analytical indicators such as iodine number , Reichert-Meißl number , saponification number , peroxide number or acid number are determined. For the qualitative and quantitative determination of individual fat components, chromatographic methods are preferably used. The fatty acid distribution can be determined using gas chromatography . Accompanying fat substances such as zoo or phytosterols or lipophilic vitamins are also determined by gas chromatography or HPLC . For the reliable identification of individual components of the fats, mass spectrometry is mostly used in combination with gas chromatography or HPLC. The German Society for Fat Science has already defined more than 400 analysis methods, including methods for identifying the authenticity of virgin olive oil or the determination of degradation products in used deep-fryer fats .
Accompanying fat
The accompanying fat substances include:
Fat spoilage
Fats are perishable; they can change chemically, especially through light, higher temperatures, atmospheric oxygen, water and microbes. As a rule, the double bonds or the ester bonds are affected when spoilage occurs, making them rancid and possibly harmful to health. It is advantageous to protect fats by storing them in a cool, dry place that is inaccessible to air.
Fresh fats generally contain few free, unesterified acids. Moisture and exposure to light and microorganisms saponify fats over time. You get pissed off and rancid. An indication for this is the acid number SZ (or neutralization number NZ), which indicates how many milligrams of potassium hydroxide are required to neutralize the free acids contained in one gram of fat.
Related topics
Oil paints
In art history, oils play a very important role as binders . Mixed with color pigments , these oil colors were of decisive importance for the development of painting (see also: Oil painting ). Vegetable fats are also used as a varnish (protective coating after painting).
Fat in art
Fat was a material used more frequently by the artist Joseph Beuys to symbolize energy stored within an artistic object or a room installation. The most famous objects are the fat corner and the fat stool .
Grease trap
Typically, grease traps are used in butchers , slaughterhouses , deep-frying and large kitchens . They are always used when fats and oils of organic origin are to be retained from the waste water. The dirty water is led into the grease separator via an integrated baffle plate, which leads to a reduction in the flow speed and a uniform flow distribution. The separation of the separable light matter (fat) and suspended matter (sludge) from dirty water is achieved solely by the effect of gravity. With a coalescence separator , finer oils and fats can also be separated.
See also
- Grasas y Aceites , trade journal
literature
- FD Gunstone, JL Harwood, FB Padley: The Lipid Handbook. Chapman and Hall, London / New York 1986, ISBN 0-412-24480-2 .
- M. Bockisch: Dietary fats and oils. (= Handbook of Food Technology ). Verlag E. Ulmer, Stuttgart 1993, ISBN 3-8001-5817-5 .
Web links
- Fats in nutritional practice: Consequences of increased fat consumption, blood lipids HDL and LDL, cholesterol, reduction in fat intake
- German Society for Fat Science
- Euro Fed Lipid - The European Federation for the Science and Technology of Lipids
Individual evidence
- ↑ a b Council Directive 90/496 / EEC of September 24, 1990 on the nutrition labeling of foods (PDF)
- ^ The dictionary of origin (= Der Duden in twelve volumes . Volume 7 ). Reprint of the 2nd edition. Dudenverlag, Mannheim 1997 ( pp. 185 , 182 ). See also Friedrich Kluge : Etymological dictionary of the German language . 7th edition. Trübner, Strasbourg 1910 ( p. 134 ).
- ↑ Stat. Federal Office, Wiesbaden, Manufacturing Industry, Series 4, Series 4.1.1, January 2008.
- ↑ Federal Statistical Office Wiesbaden, Manufacturing 2007, Series 4, Series 3.1.
- ^ Siegfried Hauptmann : Organic chemistry. 2nd, revised edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, ISBN 3-342-00280-8 , pp. 653-654.
- ↑ F. Laugerette et al.: CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. In: J Clin Invest. 115, No. 11, 2005, pp. 3177-3184. PMC 1265871 (free full text)
- ↑ A. El-Yassimi et al .: Linoleic Acid Induces Calcium Signaling, Src Kinase Phosphorylation, and Neurotransmitter Release in Mouse CD36-positive Gustatory Cells. In: J Biol Chem . 283, No. 19, 2008, pp. 12949-12959. doi: 10.1074 / jbc.M707478200
- ^ Journal for practical chemistry . 68, 1.
- ↑ reactome.org: Triacylglyceride biosynthesis. doi : 10.3180 / REACT_1190.2
- ↑ D'Eustachio / reactome.org: Hormone-sensitive lipase (HSL) -mediated triacylglycerol hydrolysis ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. . doi : 10.3180 / REACT_494.1 .
- ^ H. Pardun: Analysis of dietary fats. Paul Parey Verlag, Berlin / Hamburg 1976, ISBN 3-489-78814-1 .
- ^ W. Christie: Lipid Analysis. 2nd Edition. Pergamon Press, Oxford UK 1982, ISBN 0-08-023791-6 .
- ↑ Takayuki Shibamoto (Ed.): Lpid Chromatographic Analysis. (= Chromatographic Science Series. Vol. 65). Marcel Dekker, New York / Basel / Hong Kong 1994, ISBN 0-8247-8941-5 .
- ^ Robert C. Murphy: Mass Spectrometry of Lipids. (= Handbook of Lipid Research. Vol. 7). Pergamon Press, New York / London 1993, ISBN 0-306-44361-9 .
- ↑ Hans Kaunitz: Studies on the nutrition of rats with highly oxidized fats. In: Naunyn-Schmiedebergs Archive for Experimental Pathology and Pharmacology. 220 (1-2), 1953, p. 16.
- ↑ Key figures from fats , TomChemie.de


