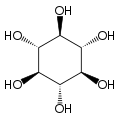
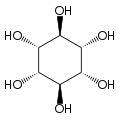
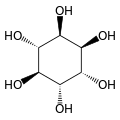
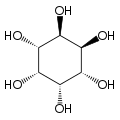
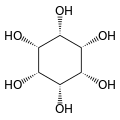
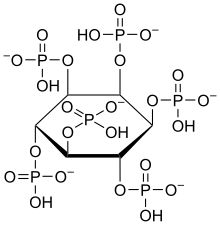
Inositol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Structural formula of myo- inositol | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Inositol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 12 O 6 | |||||||||||||||
| Brief description |
white, crystalline, odorless solid with a sweetish taste |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 180.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.75 g cm −3 |
|||||||||||||||
| Melting point |
223-225 ° C |
|||||||||||||||
| solubility |
143 g l −1 (19 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Inositol ( the inositol; also, especially in English : inositol ; outdated: "muscle sugar") is the common name for cyclohexane hexol , a hexavalent cyclic alcohol .
Although inositol has the common name "muscle sugar", it is not a carbohydrate , as it has no carbonyl group and therefore cannot form a cyclic hemiacetal . It only fulfills the original criterion of a carbohydrate ( hydrated carbon ), namely that its molecular formula is C n (H 2 O) n or that inositol is an isomer (the same molecular formula ) to glucose and fructose.
The variant inositol , which actually originates from the Anglo-American language area, has become naturalized in the natural sciences, especially in compound terms it is the far more common variant.
In the past, inositol was assigned to the B vitamins and partly (like adenosine monophosphate ) was called "vitamin B8".
Isomerism
Depending on the position of the hydroxyl groups , nine stereoisomers are possible, one of which is a pair of enantiomers ( chiro- inositol). In the most common form in nature, the hydroxyl groups on carbon atoms 1, 2, 3 and 5 are on the same side of the ring and the hydroxyl groups on C 4 and C 6 are on the opposite side. This form has the systematic name cyclohexan- cis -1,2,3,5- trans -4,6 -hexol, the common name myo-inositol and occurs in many animal and vegetable tissues.
The common names of the other rare isomers are: scyllo -, D -chiro -, L -chiro -, muco -, neo -, allo -, epi - and cis -inositol.
All isomers have a sweet taste.
Occurrence and meaning
In the body, inositol is usually esterified with phosphates . It was previously assigned to the B vitamins . Due to the fact that the human body can produce inositol itself from glucose , this classification is now considered out of date. For example, each of the two human kidneys produces around 2 g of inositol per day, a total of 4 g / d. This is a multiple of the amount that is consumed daily with food (225 to 1800 mg / d, an average of 900 mg / d with a standard diet of 2500 kcal / d). Other body cells can also produce inositol and contribute slightly to the supply. The highest inositol concentrations are measured in the brain , where it functions as a secondary messenger substance and part of neurotransmitters . For micro-organisms inositol acts as a growth factor and is there as Bios I called. In almost all higher plants it occurs as a component of sphingolipids . In fungi , bacteria and some higher plants, alcohol occurs mainly as phytic acid ( inositol hexaphosphoric acid ) for phosphate storage.
Secondary messenger substance
The 1D- myo- inositol-1,4,5-trisphosphate (simplified inositol trisphosphate , IP 3 ) and other phosphoinositols play an important role as a secondary messenger substance in the signal transmission in cells. It is released by phospholipase- mediated hydrolytic cleavage of the phospholipid phosphatidylinositol-4,5-bisphosphate (PIP 2 ) - into a 1,2-diacylglycerol (DAG) and IP 3 . IP 3 is able to influence the cell metabolism, for example it causes the Ca 2+ concentration to rise within the cell.
See also:
- Signaling pathways of insulin
- Inositol 1,4,5-triphosphate (IP 3 ) as a secondary messenger substance
- Function of phospholipase C , signal transduction in protein kinase C.
- Endoplasmic reticulum as calcium storage
Extraction
myo- inositol is obtained by hydrolysis of phytic acid, which in turn can be isolated from corn steep water :
use
Inositol is commercially available as a dietary supplement for humans or horses. Due to its visual similarity, ready availability, and low price, inositol powder is often used to stretch cocaine or methamphetamine . It can also be found in various energy drinks .
Inositol is used in the treatment of Polycystic Ovary Syndrome (PCOS). Studies have shown that inositol can help reduce insulin resistance and hyperinsulinism in PCOS and prediabetes and obesity .
Historical
The German chemist Josef Scherer , a former student of Justus Liebig and a professor in Würzburg , isolated inositol from muscle tissue and in his publication in 1850 suggested the name "inositol" after the Greek "is", genitive "inos" , for tendon, muscle.
proof
Inositol can be detected by oxidation with nitric acid , with rhodizonic acid C 6 H 2 O 6 , which forms a characteristic red barium salt. This reaction, published in 1852 by Josef Scherer, in which the molecular structure, the carbon six-ring, is retained, is also called the Scherer reaction after its discoverer.
Individual evidence
- ↑ a b c d e f data sheet inositol at AlfaAesar, accessed on February 17, 2013 ( PDF )(JavaScript required) .
- ↑ Myo-Inositol data sheet (PDF) from Merck , accessed on January 11, 2017.
- ↑ "Vitamin B8" on amazon.de
- ↑ External identifiers or database links to myo-Inositol : CAS number: 87-89-8, EC number: 201-781-2, ECHA InfoCard: 100.001.620 , ChemSpider : 10239179 , DrugBank : DB03106 , Wikidata : Q15628176 .
- ↑ External identifiers or database links for scyllo-inositol : CAS number: 488-59-5, EC number: 610-437-4, ECHA InfoCard: 100.113.358 , ChemSpider : 10254646 , DrugBank : DB03106 , Wikidata : Q3023527 .
- ↑ External identifiers or database links for muco-inositol : CAS number: 488-55-1, EC number: 207-681-5, ECHA InfoCard: 100.006.983 , ChemSpider : 16736990 , Wikidata : Q743661 .
- ↑ External identifiers of or database links to D -chiro-inositol : CAS number: 643-12-9, EC number: 211-394-0, ECHA InfoCard: 100.010.359 , ChemSpider : 10254647 , Wikidata : Q3011024 .
- ↑ External identifiers or database links for L- chiro-inositol : CAS number: 551-72-4, EC number: 209-000-7, ECHA InfoCard: 100.008.183 , ChemSpider : 10199754 , Wikidata : Q3205874 .
- ↑ External identifiers or database links for neo-inositol : CAS number: 488-54-0, ChemSpider : 10199749 , Wikidata : Q3347078 .
- ↑ External identifiers of or database links to allo-inositol : CAS number: 643-10-7, EC number: 211-393-5, ECHA InfoCard: 100.010.358 , PubChem : 135058350 , ChemSpider : 16736991 , Wikidata : Q2838375 .
- ↑ External identifiers or database links for epi-Inositol : CAS number: 488-58-4, EC number: 207-682-0, ECHA InfoCard: 100.006.984 , ChemSpider : 10254648 , Wikidata : Q3589114 .
- ↑ External identifiers or database links for cis-inositol : CAS number: 576-63-6, ChemSpider : 16736992 , Wikidata : Q2974313 .
- ^ Marine L. Croze, Christophe O. Soulage: Potential role and therapeutic interests of myo-inositol in metabolic diseases . In: Biochemistry . tape 95 , no. October 10 , 2013, ISSN 1638-6183 , p. 1811-1827 , doi : 10.1016 / j.biochi.2013.05.011 , PMID 23764390 .
- ^ Wissenschaft-Online-Lexika: Entry on inositols in the Lexikon der Chemie , accessed June 4, 2008.
- ^ National Drug Intelligence Center: South Carolina Drug Threat Assessment: Methamphetamine , December 2001
- ↑ a b V. Unfer, JE Nestler, ZA Kamenov, N. Prapas, F. Facchinett: Effects of inositol (s) in Women with PCOS: A Systematic Review of Randomized Controlled Trials . In: International Journal of Endocrinology . tape 2016 , 2016, p. 1849162 , doi : 10.1155 / 2016/1849162 , PMID 27843451 , PMC 5097808 (free full text).
- ↑ C. Bañuls, S. Rovira-Llopis, S. López-Doménech, S. Veses, VM Víctor, M. Rocha, A. Hernández-Mijares: Effect of consumption of a carob pod inositol-enriched beverage on insulin sensitivity and inflammation in middle-aged prediabetic subjects . In: Food & Function . tape 7 , no. October 10 , 2016, p. 4379-4387 , doi : 10.1039 / c6fo01021k , PMID 27713964 .
- ↑ Josef Scherer: About a new type of sugar obtained from muscle meat . In: Friedrich Wöhler, Justus Liebig (Ed.): Annals of Chemistry and Pharmacy . tape 73 , no. 3 . Christian Friedrich Winter, 1850, ISSN 0075-4617 , p. 322-328 , doi : 10.1002 / jlac.18500730303 ( hathitrust.org ).
- ↑ AKM Shamsuddin, Guang-Yu Yang: Inositol & its Phosphates: basic science to practical applications . Bentham Science Publishers, Sharjah 2015, ISBN 978-1-68108-008-6 ( limited preview in Google Book Search).
- ↑ Josef Scherer: About the inositol . In: Friedrich Wöhler, Justus Liebig, Hermann Kopp (Hrsg.): Annalen der Chemie und Pharmacie . tape 81 , no. 3 . Christian Friedrich Winter, 1852, ISSN 0075-4617 , p. 375-375 , doi : 10.1002 / jlac.18520810313 ( archive.org ).
- ^ SJ Angyal: Inositols . Chemistry, Physiology, Pathology, Methods. In: WH Sebrell Jr., Robert S. Harris (Eds.): The Vitamins . second ed. volume III . Academic Press, Elsevier, New York and London 1971, ISBN 978-0-12-633763-1 , pp. 340-415 , doi : 10.1016 / b978-0-12-633763-1.50010-5 (English, limited preview in Google book search).