Wagner-Meerwein rearrangement
The Wagner-Meerwein rearrangement is a name reaction in organic chemistry . It was discovered in Kazan (Russia) in 1899 by Georg Wagner (Егор Егорович Вагнер) and examined by Hans Meerwein from 1914 . It is an intramolecular rearrangement of atoms or groups of molecules in carbenium ions .
Overview reaction
It is an acid- catalyzed , nucleophilic [1,2] rearrangement of the carbon structure via carbenium ions. This type of rearrangement is also called anionotropic rearrangement . Since a σ-bond moves during the reaction, it is a sigmatropic process. R 1 and R 2 are organyl radicals or hydrogen atoms and R 3 are different organyl radicals.
mechanism
The rearrangement step shown in the overview reaction cannot be carried out separately. It is always part of reaction sequences. Reactions in which these rearrangements take place essentially consist of three steps:
- First a carbenium ion is generated (e.g. in elimination reactions or in nucleophilic substitutions )
- This carbenium ion is stabilized in the next step. This is the reaction sequence in which the [1,2] rearrangement takes place.
- In the last step, for example, the elimination of a proton or a nucleophilic attack creates the reaction product.
The driving force behind such rearrangements is the formation of more stable carbenium ions. In the stabilization of carbenium ions, the positive charges are better stabilized by having more alkyl residues in the vicinity (see hyperconjugation ). I.e. tertiary carbenium ions are more stable than secondary or primary ones.
requirements
In order for a rearrangement to take place, a good leaving group (e.g. protonatable hydroxyl group or halide ) must be present on a carbon atom that is itself bound to a more highly substituted carbon atom.
Rearrangement of an alkyl group
The mechanism of the rearrangement of an alkyl group using the example of an elimination reaction:
By splitting off a halide from a haloalkane 1 , a secondary carbenium ion 2 is generated. Since this is less stable compared to tertiary carbenium ions, an alkyl group (here marked in blue) is rearranged . This creates intermediate level 3 . In the last step, this tertiary carbenium ion 3 is deprotonated and the alkene 4 is formed .
Hydride shift
The following example (isomerization of a haloalkane) illustrates the [1,2] rearrangement of a hydride ion . The reaction is a nucleophilic substitution in which a primary reacts to a secondary haloalkane. This is also called isomerization .
The hydride shift is basically analogous to the rearrangement of an alkyl group (see above). By using aluminum bromide in catalytic amounts, the leaving group (in this case bromide ) is split off and a primary carbenium ion 2 is generated from the bromoalkane 1 . A more highly substituted carbon atom is bound to the positively charged carbon atom, namely a secondary one. Thus a Wagner-Meerwein rearrangement can take place. The carbenium ion 2 reacts to intermediate 3 by rearranging a hydride ion (here marked in blue). This creates a secondary carbenium ion 3 , which is attacked by the nucleophilic leaving group and reacts further to form the isomer of the starting compound, a secondary bromoalkane 4 .
use
The synthetic potential of the Wagner-Meerwein rearrangement is naturally low and otherwise it is often more of an undesirable side reaction in the laboratory - for example in elimination reactions. However, it has a special meaning in terpene chemistry .
Terpene chemistry
The Wagner-Meerwein rearrangement is helpful in the dehydration of tetrahydrofurfuryl alcohol to dihydropyran . Furthermore, Wagner-Meerwein rearrangements occur in the biosynthesis of cholesterol .
| Tip: In the case of rearrangement reactions with more complex molecules, it is helpful to provide the carbon atoms of the starting materials and products with numbers. This highlights the differences in the connections. The numbers do not refer to the numbering of the root system of the IUPAC - nomenclature . |
It also plays a major role in the production of camphenes . Camphene is an intermediate product of the synthesis for taste and odor substances.
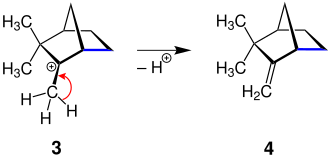
This is an acid- catalyzed dehydration of isoborneol to camphenene (cf. the elimination reaction for the rearrangement of alkyl radicals, see above). It should be noted that the product in the form shown here has been rotated in the room compared to the educt for better clarity. Note the numbering of the carbon atoms here. The blue marked bond between two carbon atoms represents the migration of the organyl residues.
The mechanism looks like this:
First, the isoborneol ( 1 ) is dehydrated, whereby the carbenium ion 2 is generated. This secondary cation is more unstable than the tertiary cation 3 . This is the reaction sequence in which the Wagner-Meerwein rearrangement takes place. To do this, the ring system is broken and a new bond is created between two carbon atoms - an alkyl group is rearranged. The connection 3 is shown here in two ways. By rotating in three-dimensional space, the structure on the right was created from the structure on the left.
A hydrogen atom is eliminated from the stabilized carbenium ion 3 and camphene ( 4 ) with the double bond results .
Others
The Wagner-Meerwein rearrangement is similar to the pinacol rearrangement . This is also an anionotropic skeletal rearrangement of the carbenium ion and specifically treats glycols , which react to ketones by splitting off water . Thus, one can understand the pinacol rearrangement as a special form of the Wagner-Meerwein rearrangement.
Individual evidence
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. An introduction to organic chemistry . Verlag Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 205 .
- ^ Georg Wagner: J. Russ. Phys. Chem. Soc. 31, 1899, p. 690.
- ↑ Hans Meerwein : About the reaction mechanism of the conversion of borneol into camphene. [Third communication on pinacolin deposits.] In: Justus Liebigs Annalen der Chemie . tape 405 , no. 2 , 1914, p. 129-175 , doi : 10.1002 / jlac.19144050202 .
- ↑ H. Meerwein, Konrad van Emster: About the equilibrium isomerism between bornyl chloride, isobornyl chloride and camphene chlorohydrate , reports of the German Chemical Society, Volume 55, 1922, p. 2500
- ↑ Reinhard Brückner: reaction mechanisms: organic reactions, stereochemistry, modern synthesis methods . 3rd, updated and revised. Edition. Elsevier, Spektrum, Akad. Verlag, Munich / Heidelberg 2004, ISBN 3-8274-1579-9 , pp. 589-595 .
- ↑ a b c Thomas Laue, Andreas Plagens: Name and catchword reactions of organic chemistry . 5th, through. Ed. Teubner, Wiesbaden 2006, ISBN 3-8351-0091-2 , p. 336-338 .
- ↑ a b H. Becker et. al .: Organikum. Organic-chemical basic internship . 22., completely revised. and updates Edition. Wiley-VCH, Weinheim [u. a.] 2004, ISBN 3-527-31148-3 , pp. 666 .
- ↑ Stefan Bräse, Jan Bülle, Aloys Hüttermann: Organic and bio-organic chemistry. The basic knowledge for master's and diploma exams . Wiley-VCH-Verl, Weinheim 2008, ISBN 978-3-527-32012-7 , pp. 552 .
- ↑ J. Clayden, N. Greeves, S. Warren, P. Wothers: Organic chemistry . Oxford University Press, Oxford / New York 2001, ISBN 0-19-850347-4 , pp. 981-982 .
literature
- LM Harwood: Basic Texts Chemistry: Polar Rearrangements. Wiley-VCH, Weinheim 1997, ISBN 3-527-29291-8 .
- Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , pp. 592–602.
- J. Clayden, N. Greeves, S. Warren, P. Wothers: Organic Chemistry. Oxford University Press, 2001, ISBN 0-19-850346-6 , pp. 981-982.