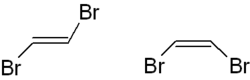Nomenclature (chemistry)
In chemistry, nomenclature is understood to be the most systematic and internationally uniform naming possible for chemical substances. Today it is important that a connection name is unique and only leads to a single structural formula . For example, the term “ ethanol ” only refers to the compound CH 3 –CH 2 –OH and no other compound . Conversely, chemical compounds do not have a clear name, e.g. For example, the compound CH 3 –CH 2 –OH can be referred to as “ethanol” and “ethyl alcohol” according to various nomenclature systems.
history
Until the 18th century, the names of chemical substances were very inconsistent. The book Méthode de nomenclature chimique by Louis Bernard Guyton de Morveau , Antoine Laurent de Lavoisier , Claude Louis Berthollet and Antoine François de Fourcroy in 1787 represented an important step towards systematisation . Jöns Jakob Berzelius introduced chemical sign language around 1825 with letters for chemical elements a. In 1860 a committee headed by Friedrich August Kekulé proposed an international designation system for organic compounds. In 1919 the International Union of Pure and Applied Chemistry (IUPAC) was founded. Since then, she has considered setting international standards for chemical nomenclature to be her main task.
The IUPAC nomenclature
In order to standardize the designations for chemical compounds, there are the internationally binding guidelines of the IUPAC (International Union of Pure and Applied Chemistry) and the IUBMB (International Union of Biochemistry and Molecular Biology) as well as their Joint Commission on Biochemical Nomenclature, which is set up as a compensation committee . These regulate the use of English. The names in other languages are transferred accordingly by the national chemists' associations. In the German-speaking area, for example, the German Central Committee for Chemistry, under the management of the Gesellschaft Deutscher Chemiker ( GDCh ), is responsible for implementation in agreement with the national IUPAC member societies in Switzerland and Austria. The IUPAC itself also uses many English names in its element lists instead of the basic element abbreviations (e.g. potassium, sodium, tungsten, mercury). The needs of different languages and even English itself are expressly recognized by the IUPAC. Particularly stringent nomenclature regulations were necessary, especially for index works for chemical substances such as Beilstein's Handbook of Organic Chemistry and Chemical Abstracts , since their system for finding entries until the introduction of electronic research was mainly based on this.
Since the systematic designation of chemical compounds according to these rules is often very complicated, a large number of traditional names or newly created, recognized short names are still used by chemists in everyday use and in scientific publications. The IUPAC distinguishes between trivial names which no relation to the systematic nomenclature have (z. B. water , urea or Glauber's salt ), semi systematic name or semi Trivial names for at least a part of a systematic name using (. As carbon dioxide instead of carbon material dioxide, trityl for the triphenylmethyl group or glycerine for propane-1,2,3-triol) and the systematic name already mentioned. Also for the invention of new trivial names, e.g. B. of newly discovered natural substances , there are IUPAC-compliant rules.
Furthermore, the national customs prevail with the element names and even the IUPAC name stems do not consistently correspond to the name that is authoritative for the formula abbreviation (example Hg = hydrargyrum, German mercury, IUPAC root “mercur” such as English mercury and Latin Mercurius).
Element names and symbols
The names of the chemical elements are determined by the discoverers. For unknown or new elements that have not yet been given a name, there are systematic element names that are derived from the atomic number . The periodic table of the elements offers a systematic arrangement of the elements according to their electronic configuration . For some elements there are old German names that have been adapted in several revisions by the IUPAC to those in English. This primarily element names in which the letters k and z to c were exchanged. Examples are calcium - calcium, silicon - silicon or cobalt - cobalt. But also some other spellings such as iodine which was changed to iodine or bismuth to bismuth were changed. While the new names are predominantly used in chemistry, the old names are still used in many other areas and in the general language area.
For each element there is an abbreviation consisting of one to three letters ( element symbol ) . The element symbols are internationally valid, so they are also represented in Japanese using Latin letters, for example.
If you want to designate a certain isotope of an element, you put its mass number in front of the element symbol, for example 12 C for the carbon-12 isotope, 235 U for uranium 235 etc. The heavy isotopes of hydrogen , 2 H ( deuterium ) and 3 H ( tritium ), with D or T also have their own element symbol.
In order to name connections between different elements, the element names are partially modified and given suffixes. For this purpose, the name stem , which is derived from the Latin or Greek element names, is used. For example, the oxygen in the compound aluminum ox id (Al 2 O 3 ) is indicated by its stem (ox) and the ending -id .
Number prefixes in chemical names
If a type of atom or atom group occurs more than once in a molecule, the number is indicated by a corresponding numerical prefix (prefix), which is derived from the Greek numerals and precedes the name of the corresponding atom or group of atoms. Exceptions are nona and undeca , which are derived from Latin.
|
|
|
The prefixes mono and di are only used for one and two. In connection with other numerals (21, 101 etc.) hen and do are used. The formation of the complete numeral is done from back to front .
| Do- | -nonaconta- | -tetracta- | -kilia |
|---|---|---|---|
| 2 | 90 | 400 | 1000 |
| 1492 = Dononacontatetractakilia | |||
Examples:
- P 4 S 7 tetra phosphorus hepta sulfide
- SO 3 sulfur tri oxide
- CH 2 Cl 2 Di chloromethane
Omission of number prefixes
In the case of metal compounds, only the valency or oxidation number that the metal has in this compound ( ionic bond ) is mentioned: z. E.g .: CrO 3 chromium (VI) oxide, read chromium six oxide instead of chromium trioxide ( Stock's nomenclature ). The valence or oxidation number is given in Roman numerals. If the name of a connection remains unique as a result, the value can be omitted. So there are z. B. only a single oxide of aluminum, namely Al 2 O 3 , which is why you can simply write aluminum oxide instead of aluminum (III) oxide.
Very often the prefix mono- is omitted, e.g. , NaCl = sodium chloride and no sodium mono chloride.
Alternative number prefixes
If there are several identical groups for which the use of the above prefixes would be ambiguous, the following prefixes derived from the Greek are used. From four onwards, the simple number prefix is used together with the ending -kis .
| number | Prefix |
|---|---|
| 1 | hen |
| 2 | to |
| 3 | tris |
| 4th | tetrakis |
| 5 | pentakis |
| 6th | hexakis |
| ... | |
| 21st | henikosakis |
Examples:
- Ca 5 F (PO 4 ) 3 Pentacalciumfluorid tris phosphate - By using the prefix tris it is immediately clear that it is not the triphosphate group [P 3 O 10 ] 5− , but three phosphate groups [PO 4 ] 3− .
- 5,6- bis (1,1-dimethylpropyl) undecane - the use of the prefix bis immediately shows that there are two identical 1,1-dimethylpropyl substituents.
The following prefixes, which are derived from the Latin numerals, are used to directly link identical units :
| number | Prefix |
|---|---|
| 2 | bi |
| 3 | ter |
| 4th | quater |
| 5 | quinque |
| 6th | sexi |
| 7th | septi |
| etc. |
Example:
- C 6 H 5 –C 6 H 5 is called Bi phenyl (and not diphenyl or bisphenyl).
Inorganic chemistry
Formulas of inorganic compounds
When writing formulas of chemical compounds , one essentially follows the electronegativity scale of the chemical elements. You always start with the more electropositive connection partner , which is why you write AgCl, Al 2 O 3 , PCl 5 and not the other way around. Hydrogen compounds are an exception to this rule.
Hydrogen compounds
Hydrogen atoms are written last in the formulas (NH 3 , SiH 4 etc.). However, if it is acidic hydrogen (i.e. the compound reacts acidic in aqueous solution), write the hydrogen at the beginning of the formula (HF, HCl, HBr, HI, H 2 O, H 2 O 2 , H 2 S, H 2 Se, H 2 Te). When naming these compounds, for example, in addition to the name “hydrogen fluoride” for HF or “hydrogen chloride ” for HCl, the name hydrogen fluoride or hydrogen chloride , which is much more common in the laboratory, is also used. The latter are to be preferred due to the clearer designation, since these compounds are not salts with the corresponding anions, but rather, due to the high electronegativity numbers , only highly partially charged dipole compounds in which there is a strongly polar covalent bond (atomic bond). In addition, due to the clarity of the naming, it should be noted that, for example, “hydrogen fluoride” is understood to mean salts of the MHF or MF × HF type (with M = monovalent metal), so hydrogen fluoride is preferred. In the case of inorganic oxo acids, the hydrogen is also written at the beginning of the formula, although it is actually bound to oxygen, i.e. for sulfuric acid, for example, H 2 SO 4 instead of SO 2 (OH) 2 .
radical
To name the radical, the ending -yl is appended to the stem name. This applies to both organic and inorganic chemistry.
Examples: HO • : Hydroxyl (stem: Hydrox-), • CH 3 : Methyl (stem: Meth-) and R – O • : Oxyl (e.g. 2,2,6,6-tetramethylpiperidinyloxyl )
Some radicals have special names, especially when it comes to oxygen compounds.
Organic chemistry
The naming of organic compounds according to the IUPAC system is usually based on a parent system that may have additional substituents (residues). A substituent is an atom or an atom combination which replaces (substitutes) a hydrogen atom in the parent system. The name of the parent system is used unchanged for naming the compound and the names of the substituting groups are added to the parent system in a modified form ( substitutive nomenclature ).
In addition to the parent system, a descriptor named prefix is added in front of the systematic substance name in the case of corresponding compounds [e.g. B. cis -, trans -, ( E ) -, ( Z ) -, o -, m -, p -, n -, iso -, neo -, cyclo -, sec -, tert -, D -, L - , meso -, (±) -, (+) -, (-) -, ( RS ), ( R ) -, ( S ) -], which describes the configuration or the stereochemistry of the molecule. Descriptors are often used in combination with locants (e.g. O -, N -, S -, α-, β-, [3.3]) to precisely describe certain positions of atoms or bonds in order to clearly name a chemical structure.
Brackets
In order to obtain unambiguous formulas and names, parts of names and special information are put in brackets in the nomenclature. Three types of brackets are used: round (), square [], and curly {}. The use of square brackets is different in inorganic and organic chemistry. Units that occur more than once are put in round brackets, with the exception of coordination units , which are always in square brackets. In the name of organic compounds, round, square and curly brackets are used from the inside out: {[()]}, {[({[()]})]} etc. In formulas, the different order [], [() ], [{()}], [({()})], [{({()})}] are used.
In the case of incompletely isotopically substituted compounds, the item numbers and nuclide symbols are put in round brackets, for example dichloro ( 2 H 2 ) methane. In the case of specific, unselective, selectively marked, isotopically deficient or enriched compounds, the nuclide symbols with multiplication subscripts are put in square brackets, for example for [ 13 C, 2 H 2 ] methane, [ def 13 C] chloroform, [ 12 C] chloroform.
In the case of polycyclic hydrocarbons, the merging positions of substructures are inserted in square brackets, for example in benzo [ a ] anthracene .
In the case of bridged polycyclic hydrocarbons in the Von-Baeyer system , after the term cyclo, the number of carbon atoms of the two branches of the main ring, the main bridge and any secondary bridges are given in descending order in square brackets, for example bicyclo [4.4.0] decane . In the case of unsaturated bridges, the locants of the multiple bonds are given in square brackets within the bridge term.
In the case of spiro hydrocarbons, the term spiro is followed by the sums of the carbon atoms bound to the spiro atom in square brackets, for example spiro [2.4] heptane. If a formal (di) -hydrogenation has to be carried out in order to be able to realize a Spiro link at all, the additional indexed hydrogen is placed in round brackets directly behind the number relating to the Spiro link.
In the case of oligosaccharides with a free hemiacetal group, the linking locants are placed in round brackets between the individual component names, for example β-D-galactopyranosyl- (1 → 4) -α-D-glucopyranose.
Spaces and hyphens
In all places where spaces are used in English between the words of a name, there are hyphens in German in organic chemistry or the space is omitted. The use of hyphens is a bit arbitrary at times. If it's for the sake of clarity, be a little generous here.
Hyphens are used in FORMULAS and in NAMES:
- To separate locants from words or morphemes of the name. Example: but-2-en
- To separate a stereo descriptor from a name. Example: ( E ) but-2-en
- To separate symbols like µ from the rest of the formula or name.
- To structure descriptors such as cyclo , catena , triangulo , quadro , tetrahedro , octahedro , closo , nido , arachno , cis and trans as well as z. B. to separate Λ and α from the rest of the formula or the name. Locants are separated in the same way in the names of aggregates or clusters.
- To separate the symbol of the labeling nuclide from its locant in the formula of a selectively labeled compound.
- To separate locants belonging to different parts of the name. However, parentheses should be preferred.
- To separate the name of a bridging ligand from the rest of the name.
A hyphen appears after parentheses only if the closing parenthesis is followed by a locant, e.g. B. 3- (bromocarbonyl) -4- (chlorocarbonyl) -2-methyl-benzoic acid. Numerals (e.g. Tetra) are linked to the names without a hyphen.
Trunk systems
Linear chains
The simplest parent systems are linear chains of carbon atoms, in which all other bonds are saturated with hydrogen atoms. Such saturated hydrocarbons are called alkanes and they have the ending -an . For the four smallest alkanes, the names methane, ethane, propane and butane are retained, for the remaining alkanes the exact name of the compound is derived from the number of carbon atoms according to the following table . Combine the numerical word for the first decade with the numerical word for the following decades. At the end there is an n, giving the alkane-typical ending -an .
| 1 | Hen | 10 | Deca | 100 | Hecta | 1000 | Kilia | |||
| 2 | do | 20th | Cosa | 200 | Dicta | 2000 | Dilia | |||
| 3 | Tri | 30th | Triaconta | 300 | Tricta | 3000 | Trilia | |||
| 4th | Tetra | 40 | Tetraconta | 400 | Tetracta | 4000 | Tetralia | |||
| 5 | Penta | 50 | Pentaconta | 500 | Pentacta | 5000 | Pentalia | |||
| 6th | Hexa | 60 | Hexaconta | 600 | Hexacta | 6000 | Hexalia | |||
| 7th | Hepta | 70 | Heptaconta | 700 | Heptacta | 7000 | Heptalia | |||
| 8th | Octa | 80 | Octaconta | 800 | Octacta | 8000 | Octalia | |||
| 9 | Nona | 90 | Nonaconta | 900 | Nonacta | 9000 | Nonalia |
Examples:
- C 32 H 66 = Dotriacontan (Do + Triaconta + n)
- C 99 H 200 = Nonanonacontan (Nona + Nonaconta + n)
- C 403 H 808 = Tritetractan (Tri + Tetracta + n)
- C 4728 H 9458 = Octacosaheptactatetralian (Octa + Cosa + Heptacta + Tetralia + n)
- C 9999 H 20000 = Nonanonacontanonactanonalian (Nona + Nonaconta + Nonacta + nonalia + n)
There are exceptions to the naming according to the table above:
| Number of carbon atoms | connection | Surname |
|---|---|---|
| 1 | CH 4 | methane |
| 2 | C 2 H 6 | Ethane |
| 3 | C 3 H 8 | propane |
| 4th | C 4 H 10 | butane |
| 11 | C 11 H 24 | Undecane |
| 20th | C 20 H 42 | Icosan |
| 21st | C 21 H 44 | Henicosan |
If there is a double bond in the compound, the term alkenes is used and the ending -en is used instead of the ending -an . The position of the double bond is indicated by a number, see numbering below , e.g. B.
- CH 2 = CH – CH 2 –CH 3 is called But-1- en (formerly 1-But en ),
- CH 3 –CH = CH – CH 3 means but-2- ene .
For chains that contain a triple bond (= alkynes ), the ending -in is used, e.g. B.
- CH≡C-CH 2 -CH 3 is but-1- in (formerly 1-But in )
- CH 2 = CH – CH 2 –C≡C – CH 2 –CH 3 is called hept- 1-ene - 4- yne .
If there are several double or triple bonds, the multiplying prefixes di, tri, tetra, penta, hexa, hepta, ...
- CH 2 = CH – CH = CH 2 is called buta- 1,3-diene ,
- CH≡C – C≡C – C≡C – CH 3 is called hepta- 1,3,5-triyne.
Determination of the main chain in branched acyclic hydrocarbons
The main chain (stem system) is that chain which
- the largest number of multiple bonds contains
- if (1) is ambiguous: contains the larger number of carbon atoms
- if (2) is ambiguous: contains the larger number of double bonds
- if (3) is ambiguous: has the lowest locant set for the multiple bonds.
- if (4) is ambiguous: has the lowest locant set for the double bonds.
- if (5) is ambiguous: has the larger number of substituents.
- if (6) is ambiguous: has the lowest locant set for the substituents.
- if (7) is ambiguous: has the first substituent in alphabetical order.
- if (8) is ambiguous: has the lowest locant for the alphabetically first substituent.
Note on the locant set : A locant set is the enumeration of the locants such as B. 2,4 in 2,4-dimethyl-heptane. The “lowest set of locants” does not mean the smallest sum of the locants, rather the locants are compared one after the other. The smallest locant set is that which has the smaller locant at the first distinguishable position.
Cyclic systems without heteroatoms
In cyclic systems, one cycle is generally the parent system.
Monocyclic systems
If it is a monocyclic compound , the naming is the same as for linear chains, and in addition the prefix Cyclo- is placed in front, e.g. B. Cyclo hexane . The common name is retained for benzene . Monocyclic compounds with more than six carbon atoms, which have the maximum number of non-accumulated double bonds, can be referred to as (n) annulenes (n = number of carbon atoms). Cyclic systems are preferably designated according to the Hantzsch-Widman system .
Condensed polycyclic systems
In the case of condensed polycyclic hydrocarbons (i.e. the individual rings are each linked via exactly one common bond), the basic system is the component which
- has most of the rings
- has the largest ring
The following polycycles are regarded as separate systems (in increasing priority, the number of rings in brackets): pentalen (2), indene (2), naphthalene (2), azulene (2), heptalene (2), biphenylene (3) , as- indacene (3), s-indacene (3), acenaphthylene (3), fluorene (3), phenals (3), phenanthrene (3), anthracene (3), fluoranthene (4), acephenanthrylene (4), Aceanthrylene (4), triphenylene (4), pyrene (4), chrysene (4), naphthacene (4), pleiades (4), picene (5), perylene (5), pentaphene (5), pentacene (5), Tetraphenylene (5), hexaphene (6), hexacene (6), rubicene (7), coronene (7), trinaphthylene (7), heptaphene (7), heptacene (7), pyranthrene (8), ovals (10).
All other rings are put in front as prefixes, with the suffix -en being converted into -eno (e.g. Benzo cyclooctene). The type of link is indicated by numbers and letters, but this is not explained in more detail here.
When naming saturated or partially saturated derivatives of the polycycles listed above, there is the option of displaying the two additional hydrogen atoms using the item numbers and the prefix dihydro- if a double bond is omitted . Similarly, there are tetrahydro-, hexahydro- , etc. Fully saturated systems are given the prefix perhydro- . Individual hydrogen atoms are indicated by the so-called indexed H , which is placed in front of it in italics (e.g. 4 H -pyrazole).
Cyclophanes can be named according to the same rules, although they also have their own nomenclature.
Bridged polycyclic systems
For bridged polycyclic hydrocarbons (i.e. the individual rings are each linked by more than one common bond), the Von Baeyer system is used.
Spiro compounds
In spiro compounds the rings are connected by a common atom.
Nomenclature: substituents -spiro [ number of atoms in the smaller ring . Number of atoms in the larger ring ] stem name (ring size is given without spiro atom). Example: 1-bromo-3-chloro-spiro [4.5] decan-7-ol.
More complex systems
The decision of what is now to be regarded as the root system is no longer easy with more complicated connections.
Heterocycles
Unless there are trivial names, monocyclic heterocycles with up to 10 ring members are usually named according to the Hantzsch-Widman system .
In the case of condensed polycycles, heterocycles have priority over carbocycles (= rings that only consist of carbon atoms). There are also systems with trivial names for heterocycles, which are understood as separate stem systems (without ranking and incomplete):
- O-containing compounds: furan , xanthene , ...
- N-containing compounds: pyrrole , imidazole , pyrazole , ...
Otherwise, the naming of heterocycles largely follows the rules given above for cyclic systems without heteroatoms. The type and position of the heteroatoms is then indicated using the replacement nomenclature (“a” nomenclature).
Many individual compounds and groups of substances contain the ending -idin (e.g. pyrrolidine and anisidine ). In most cases, these are aromatic compounds that contain nitrogen. However, the naming does not follow a consistent system.
Substituents (residues)
A substituent can e.g. B. be a functional group , or a (smaller) parent system, such as a side chain . The names for substituents are added to the name of the root system as prefixes or endings (suffixes). The exact position of the substituent is specified by digits (see numbering below ).
If there are several prefixes, they are listed in alphabetical order.
Parent systems as substituents
If the remainder is a stem system, for example a side chain or a ring, the syllable -yl is appended to its name and the result is placed in front of it as a prefix. The naming of side chains follows the same rules as for the basic chain, with the following exceptions:
- in the case of alkanes, the ending -an is omitted
- the numbering of the side chain always starts with the link with the main chain
Examples:
- Methyl : -CH 3
- Ethyl : -CH 2 -CH 3
- Ethynyl : -C≡CH
- Prop-2-enyl (allyl): -CH 2 -CH = CH 2
- Cyclohexyl: -C 6 H 11
The names element-hydrogen groups are formed analogously from the parent compound:
- Germyl: -GeH 3
- Silyl: -SiH 3
- Trimethylsilyl: -Si (CH 3 ) 3
If, for example, to the compound propane (CH 3 -CH 2 -CH 3 ) still adheres in the center of a methane component, the resulting compound is CH 3 -CH ( CH 3 ) -CH 3 then 2-methyl propane. The compound CH 3 -CH 2 -CH ( CH 3 ) -CH 2 -CH ( CH 2 CH 3 ) -CH 2 -CH 3 is called 3-ethyl - 5-methyl heptane.
Side chains with a double connection to the basic chain have the ending -ylen ( methylene : = CH 2 ), in the case of a triple connection -ylidine ( methylidine : ≡CH).
Functional groups
The highest-ranking functional group is added as an ending (suffix), other functional groups as prefixes:
- CH 3 –CH ( OH ) –CH 3 is called propan-2- ol
- CH 3 –CH 2 –CH 2 –C ( OOH ) is called butanoic acid
- CH 3 -CH ( OH ) -CH 2 -CH ( NH 2 ) -CH 2 -CH 3 has two functional groups. The alcohol has a higher priority, therefore, is the compound 4-amino -2-hexan ol .
For the names of individual functional groups and their order of precedence, see the keyword functional group .
Common names
For some substituents there are trivial names which z. T. are also binding, such as B .:
- Phenyl: -C 6 H 5
- Benzyl: -CH 2 -C 6 H 5
- Isopropyl: -CH- (CH 3 ) 2
- Vinyl: -CH = CH 2
- u. v. m.
numbering
The master system is numbered so that the numbers received are as small as possible. CH 3 –CH 2 –CH 2 –CH (CH 3 ) –CH 3 is called 2-methylpentane and not 4-methylpentane.
As in the case of determining the main chain, the sum of the item numbers does not have to be as small as possible, but that the first distinguishable position takes on the smallest value. CH 3 -CH 2 -CH (CH 3 ) -CH (CH 3 ) -CH 2 -CH 2 -CH 2 -CH 2 -CH (CH 3 ) -CH 3 is therefore called 2,7,8-trimethyldecane and not 3 , 4,9-trimethyldecane. This also applies if item numbers appear more than once, for example with CH 2 Cl – CF 3 ( 2-chloro-1,1,1-trifluoroethane instead of 1-chloro-2,2,2-trifluoroethane).
If there is only one possible combination, the numbers can be left out (e.g. 2-methylpropane = methylpropane , since there is no other methylpropane).
If side chains have to be numbered, the connection point to the main chain is always position 1.
For naturally occurring derivatives of glycerine , the sn nomenclature applies to the numbering of the C atoms according to IUPAC .
In the case of condensed polycyclic systems, there may be binding numbering schemes that must be looked up in each case (see e.g. sterane basic structure ).
The position numbers are called locants .
Multiple occurring substituents
The multiplying prefixes di, tri, tetra, penta, hexa, hepta, ... (see above ) are used for the same groups that occur several times :
- a benzene ring with three methyl groups at positions 1, 3 and 5 is 1,3,5- tri methyl benzene ,
- a methane with four chlorine atoms called Tetra chloromethane .
- an ether with two ethyl called Di ethyl ether , etc.
If the use of di, tri, tetra etc. would be misleading, for example with identical further substituted side chains, the appropriate alternative prefixes bis, tris, tetrakis etc. must be used as described above . The prefixes bi, ter, quater, etc. are used for directly linked identical units.
example
According to the IUPAC nomenclature, for example, the compound
NH 2 -CH 2 -CH 2 -OH
received the name 2-aminoethanol.
You can get this name in the following way:
- Since the carbon atoms only have single bonds, the root is given “an” as the first ending.
- The basic chain contains two carbon atoms; this results in the root "eth". (→ "ethane").
- The functional groups are an alcohol (OH) and an amino group (NH 2 ). The alcohol group has the higher priority and takes precedence over the amino group. So “ol” is added to the end. (→ "ethanol").
- The amino group is not on the same carbon atom as the alcohol group (atom no. 1), but on the one next to it (no. 2). Therefore the place is indicated by "2-Amino".
- The combination of prefix, root and endings results in the name "2-aminoethanol".
Stereochemistry
Chiral connections
To distinguish between chiral compounds , the various forms are preceded by an italic ( R ) - or ( S ) - . Their use is determined by the Cahn-Ingold-Prelog rule (CIP rule) and its subsidiary rules. If a chiral compound is a 1: 1 mixture of enantiomers - i.e. it is a racemate - the designation ( RS ) - is placed in front. If the configuration ( R ) - or ( S ) - is uncertain or unknown, this is indicated by a (ξ) or (Ξ) - (Greek letter Xi).
For biochemical substances such as carbohydrates and amino acids , the Fischer nomenclature is also often used, which uses the prefixes D - and L - (where D and L are written as small caps ).
A (+) - or (-) - is used to distinguish the direction of rotation in optically active compounds , whereby there is no connection between the optical activity (direction of rotation) and the “direction” of chirality.
It should be noted that the different designations ( R , S or D , L and +, -) according to the different types of nomenclature cannot be derived from the other designations. Only the CIP rules are suitable for the systematic designation of compounds with several centers of chirality , the Fischer nomenclature, for example for sugar, being much more compact.
cis - trans isomers
In the cis - trans isomerism, a distinction is made in the nomenclature between compounds that have only two different substituents and compounds with more than two. The former are marked with the prefixes cis - or trans - in italics . According to IUPAC, cis - double bonds are mostly - but not consistently - marked with a preceding, italic ( Z ) ("together") and trans - double bonds with an ( E ) ("opposite"). Strictly speaking, with a ( Z ) -isomer those two substituents on adjacent atoms of a double bond on the same side of the molecule that have the highest priority in the Cahn-Ingold-Prelog system , with the ( E ) -isomer the substituents with the highest So CIP priority on opposite sides of the molecule.
The trans- 1,2-dibromoethene is shown on the left, the cis version on the right . The ( E , Z ) nomenclature can also be used here ,
- trans -1,2-dibromoethene is called ( E ) -1,2-dibromoethene,
- cis -1,2-dibromoethene as ( Z ) -1,2-dibromoethene
designated.
Here on the ring, too, the two bromine atoms are shown in the trans (left) and cis positions (“together” on one side).
This is a ( Z ) -3-methylpent-2-ene, since the higher-ranking substituents (see stereochemistry ) are on one side.
Anomers
In the case of carbohydrates , anomers are distinguished by the italic prefixes α - and β - .
biochemistry
IUPAC and IUBMB (International Union of Biochemistry and Molecular Biology) have joint guidelines for the nomenclature of enzymes . According to this nomenclature, enzyme names end with -ase and contain information about the function of the enzyme. Details under the keyword enzyme and on the IUBMB website.
In addition, a code system ( see EC numbers ) was developed in which the enzymes can be found under a four-digit code.
The nucleic acid nomenclature applies to nucleic acids .
Labeling standards outside of IUPAC regulations
The alternative NXL nomenclature is used to denote hypervalent compounds. For the practical handling of chemical substances in everyday life, there are also standards, number systems and substance databases with different orientations:
- The E numbers designate food additives approved in the European Union.
- For plastics, there is by ISO standard established abbreviation . They are used in recycling codes .
- The CAS numbers are unique identifiers for chemical substances and mixtures in the form of grouped numbers. As of April 2018, the CAS database has around 140 million entries.
- The internationally valid UN numbers are used for dangerous goods . They are assigned to individual substances or groups with the same risk and are important in the logistics chain and for rescue workers.
- Substances that are traded in the EU have EC numbers . These are an important regulatory category of European chemicals law ( REACH regulation).
literature
- IUPAC, Gerlinde Kruse (Ed.): Nomenclature of Organic Chemistry - An Introduction. 1st edition. Wiley-VCH, Weinheim 1997, ISBN 3-527-29327-2 .
- Karl-Heinz Hellwich: Chemical nomenclature . GOVI-Verlag, Eschborn 2002, ISBN 3-7741-0815-3 .
- D. Hellwinkel: The systematic nomenclature of organic chemistry. An instruction manual . 5., corr., Exp. u. supplementary edition. Springer, Berlin 2005, ISBN 3-540-26411-6 .
- Martin Negwer, Hans-Georg Scharnow: Organic chemical drugs and their synonymes, Vol. 1, 1-4138, Wiley-VCH-Verlag, Weinheim 2007, ISBN 978-3-527-31939-8
- Wolfgang Holland: The nomenclature in organic chemistry . VEB German publishing house for basic industry, Leipzig 1973, DNB 730400123 .
- Philipp Fresenius, Klaus Görlitzer: Organic-chemical nomenclature: Basics · Rules · Examples. 4th revised and expanded edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 1998, ISBN 3-8047-1588-5 .
- Ursula Bünzli-Trepp: Nomenclature of organic chemistry, organometallic chemistry and coordination chemistry. Logos Verlag, Berlin 2001, ISBN 3-89722-682-0 .
- Wolfgang Liebscher: Handbook for the application of the nomenclature of organic-chemical compounds. Akademie-Verlag, Berlin 1979, DNB 790313952 .
- Wolfgang Liebscher, Ekkehard Fluck (ed.): The systematic nomenclature of inorganic chemistry. Springer-Verlag, 2013, ISBN 978-3-642-58368-1 , doi : 10.1007 / 978-3-642-58368-1 , 388 pages.
Web links
- Nomenclature (PDF from the Institute for Analytical Chemistry at the University of Regensburg ; 55 kB)
English
- IUPAC website
- IUBMB website
- IUPAC Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. (English)
- IUPAC rules on nomenclature in organic chemistry
- IUPAC nomenclature in stereochemistry
- Comparison of chemical names generated with nomenclature software and published by authors. Gernot A. Eller: Improving the Quality of Published Chemical Names with Nomenclature Software. In: Molecules . 2006, 11, pp. 915–928 (online article in English) (PDF; 103 kB)
Individual evidence
- ^ IUPAC Nomenclature of Organic Chemistry, Introduction, R-0.1 Conventions .
- ^ IUPAC Nomenclature of Organic Chemistry, R-0.2.3 Names. .
- ↑ Table 11 Basic numerical terms (multiplying affixes). IUPAC Nomenclature of Organic Chemistry, Recommendations 1993.
- ↑ a b c d e f Wolfgang Liebscher, Ekkehard Fluck: The systematic nomenclature of inorganic chemistry . Springer-Verlag, 2013, ISBN 978-3-642-58368-1 , p. 55 ( limited preview in Google Book search).
- ↑ acdlabs.com: R-0.1.5 Enclosing marks , accessed June 11, 2017.
- ^ GJ Leigh: Principles of Chemical Nomenclature A Guide to IUPAC Recommendations . Royal Society of Chemistry, 2011, ISBN 978-1-84973-007-5 , pp. 55 ( limited preview in Google Book search).
- ↑ IUPAC: nomenclature of organic compounds ( Memento of the original from March 28, 2017 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Wolfgang Liebscher, Ekkehard Fluck: The systematic nomenclature of inorganic chemistry . Springer-Verlag, 1998, ISBN 978-3-540-63097-5 , pp. 54 ( limited preview in Google Book search).
- ↑ Dieter Hellwinkel: The systematic nomenclature of organic chemistry - instructions for use . Springer-Verlag, 2013, ISBN 978-3-662-06684-3 , pp. 3 ( limited preview in Google Book Search).
- ↑ Wolfgang Liebscher, Ekkehard Fluck: The systematic nomenclature of inorganic chemistry . Springer-Verlag, 1998, ISBN 978-3-540-63097-5 , pp. 63–66 ( limited preview in Google Book search).
- ↑ Gerlinde Kruse: Nomenclature of Organic Chemistry: An Introduction . Wiley-VCH, 1997, ISBN 3-527-29327-2 , pp. 3.85 .
- ^ IUPAC : Nomenclature of Lipids: Recommendations Lip-1 and Lip-2. .
- ↑ Chemical Substances - CAS REGISTRY. In: support.cas.org. Retrieved April 2, 2018 .