Fluoranthene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
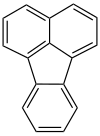
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Fluoranthene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 16 H 10 | |||||||||||||||
| Brief description |
yellow crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 202.26 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
105-110 ° C |
|||||||||||||||
| boiling point |
384 ° C |
|||||||||||||||
| Vapor pressure |
6.5 · 10 −4 Pa (25 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : persistent, bioaccumulative and toxic ( PBT ), very persistent and very bioaccumulative ( vPvB ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Fluoranthene is an unsaturated cyclic hydrocarbon . Under standard conditions, fluoranthene is a yellowish to green, crystalline solid. It occurs in coal tar and is one of the polycyclic aromatic hydrocarbons . Fluoranthene is an intermediate in the manufacture of drugs and pharmaceuticals.
The term fluoranthene is derived from the fluorescence properties of the substance and not from fluorine atoms in the molecule.
Biological importance
Fluoranthene is metabolized in the human body to 2-methylfluoranthene and 3-methylfluoranthene and their other breakdown products.
safety instructions
Fluoranthene is harmful if swallowed. In tests with animal organisms, bacteria and human cells, mutagenic and tumorigenic effects could be observed. The acute and chronic toxicity is not yet fully known. The LD 50 for oral administration to rats is 2000 mg per kg of body weight, and for dermal application to rabbits 3180 mg / kg.
Fluoranthene is classified under water hazard class 2 and therefore as hazardous to water . During the decomposition, carbon dioxide and carbon monoxide can be released.
Individual evidence
- ↑ a b c d e f g Entry on fluoranthene in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Harald Bittner, Holger Gerwig: Determination of PAHs in the particle and gas phase at typical measuring locations in Saxony , accessed on February 2, 2018.
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on January 21, 2019.

