Polycyclic aromatic hydrocarbons
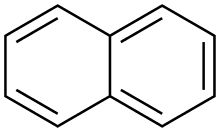
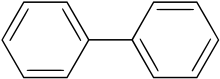
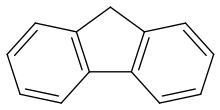


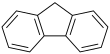
Polycyclic aromatic hydrocarbons or polycyclic aromatic hydrocarbons (short PAK or PAH from English Polycyclic Aromatic Hydrocarbons ) form a group of organic compounds that consist of at least two linked aromatic ring systems that are always in one plane. The simplest PAH is naphthalene , in which two benzene rings are fused via a common bond; this is also referred to as condensed ring systems . Fluorene is also a PAH, as both rings are rigidly connected to one another by the additional methylene unit. No PAH is biphenyl , here the two benzene rings are not fused.
These ring-shaped hydrocarbons can also carry substituents (often methyl groups ). In an extended term, derivatives with heteroatoms (primarily oxygen and nitrogen ) in the form of aldehyde , keto , carboxy and nitro groups , but also heteroaromatics , are counted among the PAHs. This results in a large number of variants within the PAK; several hundred compounds are known.
Substance data
properties
PAHs are predominantly neutral, non-polar solids. Many show fluorescence . PAHs are only slightly soluble in water; as the number of condensed rings increases, volatility and solubility (also in organic solvents ) decrease.
Numerous PAHs have been shown to be carcinogenic (carcinogenic) because they are epoxidized ( oxidized to epoxides ) during metabolism in the body and these epoxides can react with the DNA in a nucleophilic ring-opening reaction . This should not be confused with the insertion of planar hydrophobic molecules between the hydrogen-bonded base pairs of the DNA ( intercalation ).
Because of the different toxicological and physicochemical properties, a classification into low molecular weight PAHs (2–3 rings) and higher molecular weight PAHs (4–6 rings) makes sense.
PAHs are also aromatic if the number of π electrons does not correspond to the Hückel rule for aromaticity ([4n + 2] π electrons).
links
Naphthalene , a colorless solid, is the simplest PAH, which consists of two fused benzene molecules. Other important PAHs are anthracene and benzopyrene . In addition, if you include acenaphthylene , acenaphthene , fluorene , phenanthrene , fluoranthene , pyrene , Benzanthracen , coronene , ovals , tetracene , pentacene and chrysene to this group of substances. In recent years it has been possible to synthesize and characterize so-called “superacenes”. These compounds consist of a large number of fused benzene units, are very stable, have an extremely high melting point and represent a kind of precursor of graphite .
Properties of various PAHs can be found in the following list:
| Surname | Molecular formula | Molar mass / g mol −1 | M.p. / ° C | Bp / ° C | Density / g cm −3 |
|---|---|---|---|---|---|
| naphthalene | C 10 H 8 | 128.17 | 80 | 218 | 1.03 |
| Fluorene | C 13 H 10 | 166.22 | 116-117 | 295 | 1.2 |
| Phenals | C 13 H 10 | 166.22 | |||
| Anthracene | C 14 H 10 | 178.23 | 216 | 340 | 1.28 |
| Phenanthrene | C 14 H 10 | 178.23 | 101 | 340 | 0.98 |
| Pyrene | C 16 H 10 | 202.26 | 150 | 395 | 1.27 |
| Tetracene | C 18 H 12 | 228.29 | 357 | 440 | 1.35 |
| Chryses | C 18 H 12 | 228.29 | 256 | 441 | 1.27 |
| Perylene | C 20 H 12 | 252.32 | 273-278 | 350-400 subl. | 1.35 |
| Benzo [ a ] fluoranthene | C 20 H 12 | 252.32 | 144-145 | ||
| Benzo [ j ] fluoranthene | C 20 H 12 | 252.32 | 166 | ||
| Anthanthrene | C 22 H 12 | 276.33 | 264 | 547 | 1.39 |
| Pentacene | C 22 H 14 | 278.35 | <300 subl. | ||
| Pentaphs | C 22 H 14 | 278.35 | 264 | ||
| Corons | C 24 H 12 | 300.36 | 438 | 525 | |
| Hexacene | C 26 H 16 | 328.41 | 380 dec. | ||
| Heptaphs | C 30 H 18 | 378.47 | 473 | ||
| Heptacene | C 30 H 18 | 378.47 | |||
| Trinaphthylene | C 30 H 18 | 378.47 | 392 | ||
| Superphenals | C 96 H 30 | 1183.27 |
Occurrence
PAHs are a natural component of coal and petroleum . The during the coking of coal resulting tar contains high levels of PAH. Therefore, its use in road construction and z. B. banned as roofing felt since 1984. Products treated with coal tar , e.g. B. tar-bound asphalt from the time before 1984, tar paper or wood treated with tar oil (for telegraph poles or railway sleepers ) therefore contain a lot of PAHs. If the distillation of crude oil is carried out gently, only the smallest amounts of PAHs arise.
Traces of PAHs can be found in petrol and diesel fuel or heating oil . PAHs are also found in tobacco smoke and smoked, grilled and fried meat. PAHs can also accumulate in house dust on busy roads .
PAHs are an important component of interstellar matter and are detected using the methods of infrared astronomy in many areas of our Milky Way and other galaxies . The observed PAHs are mainly excited by short-wave ultraviolet radiation from nearby stars and emit in the infrared . This is why PAHs can be found in regions with strong UV radiation, such as B. in the H-II area or in star formation regions of massive stars. A very successful instrument for the detection of such PAHs was the infrared array camera (IRAC) on board the Spitzer space telescope . The 8 μm range of the infrared is dominated by PAH bands. With the infrared spectrograph from Spitzer it was possible to identify many unidentified infrared bands (UIB) already observed by the Infrared Space Observatory (ISO) in the interstellar medium as PAH emission bands .
Emergence
PAHs arise during the pyrolysis (incomplete combustion) of organic material (e.g. coal, heating oil, fuel, wood, tobacco) and must therefore be detected worldwide. The majority of PAHs today come from anthropogenic processes, but they can also be of natural origin (forest fires).
An important source with regard to the problem of contaminated sites is the extraction of coke and gas from coal. PAH-containing tars and tar oils are by-products or waste products from coking plants and former gas works and ended up in the environment through extinguishing water or processing (wood preservation ).
PAHs are also formed from humic acids through condensation reactions . In nature one observes the production of PAHs by microorganisms , fungi , plants and animals .
use
Only a few PAH individual compounds are specifically produced and used as end or intermediate products. In the chemical industry, naphthalene is used as an intermediate product mainly for azo dyes, insecticides, stabilizers, pharmaceuticals, cosmetic additives and plasticizers. It has also been used to a lesser extent as a moth control agent. 1-methylnaphthalene is used to produce the phytohormone 1-naphthylacetic acid. In the textile industry an isomer mixture of 1- and 2-methylnaphthalene was used as a solvent. Anthracene is an intermediate in paint and plastic manufacture. Some perylene derivatives are used as high quality pigments .
PAHs are a natural component of plasticizer oils based on mineral oil. These are used in soft plastics (e.g. in rubber products). Black (e.g. car tires, rubber handles on tools, synthetic leather) rubber products tend to have a higher PAH content than light-colored rubber products. However, this depends to a large extent on the type of carbon black used or on its proportion in the rubber mixture.
Solid parquet, in particular mosaic, lamellar and strip parquet, but also wooden paving, were glued to cement or asphalt screeds with tar or bituminous PAH-containing adhesives in the 1950s to 1970s .
Tar oil containing PAHs has been used extensively for impregnating wood ( carbolineum ). Products were railway sleepers, power poles and wood protection coatings. The Tar Oil Ordinance has banned it under exceptional circumstances in Germany since the 1990s . In the European Union , with immediate effect from June 1, 2009, the marketing and use of tar oils, their mixtures and wood treated with them is largely prohibited.
Harmfulness
PAHs as environmental pollutants
Because of their persistence , their toxicity and their ubiquitous presence, PAHs are of great importance as pollutants in the environment. As early as the 1980s, the American Federal Environmental Protection Agency ( EPA ) included 16 substances from the several hundred individual PAH compounds in the list of priority pollutants (see table on the right). These 16 "EPA-PAHs" have been analyzed since then mainly and as a representative for the whole group of substances.
PAHs are mainly released into the air with the exhaust gases when fossil fuels are burned. With the deposition , they are entered on and into the soil, where PAHs can be detected across the board. Locally relevant as PAH emitters are contaminated sites , e.g. B. former gas works and coking plants, tar oil processing plants (e.g. and above all railway sleepers - impregnation ) or old deposits with waste containing PAHs (e.g. ash, waste oil).
Higher-molecular PAHs with four or more rings are predominantly bound to particles in the air and in the soil. Low-molecular PAHs with two and three rings are mainly in gaseous form in the air, and dissolved in the subsoil in seepage or groundwater.
PAHs in consumer products
In a series of tests in March 2009, TÜV Rheinland found alarmingly high PAH values in rubber products such as hammer handles, bicycle horns, bathing sandals and wrist watch straps. The PAHs are absorbed by the body through long skin contact. For this reason, the Stiftung Warentest awarded the rating “poor” several times after testing trolley cases . She had detected PAK in the handles of the suitcases. The burden is so great that pulling the suitcase can become a health risk.
Plasticizers contaminated with PAH are still frequently used (as of 2017) for both cheap and high-quality products . PAHs are almost always found in synthetic leather (e.g. handbags, trimmings on clothing, belts, padding on shoulder straps) and other soft plastics (tool handles, sports bags made of smooth plastic (no fabric), etc.); the limit values mentioned below are often exceeded.
Indications for the use of PAHs are odors that do not dissipate even after intensive ventilation and long-term use. In particular, a smell like burnt rubber (“pyrolytic”), tar or mothballs or a rubbery-oily smell indicate a high PAH content. PAHs can practically not be removed even by repeated washing.
According to the findings of Stiftung Warentest, PAHs can also be found in food. She found PAHs in various types of tea, but describes the findings as "less critical" compared to other substances such as pyrrolizidine alkaloids .
Prohibitions and limit values
In the European Union, Regulation (EC) No. 1907/2006 (REACH-VO) prohibits with immediate effect
- since January 1, 2010 the use and marketing of plasticizers for (vehicle) tire production and the marketing of tires manufactured since then with per kilogram of plasticizer oil more than
- 1 mg benzo [ a ] pyrene or
- 10 mg in the sum of benzo [ a ] pyrene and another seven listed PAHs as well
- the placing on the market of consumer products and commodities with components made of plastic or rubber which, with normal or reasonably expected use, come into prolonged or repeated direct contact with the skin or oral cavity, more than 1 mg / kg, for toys and baby items 0.5 mg / kg contain one of the eight listed PAHs and were not placed on the market for the first time until December 27, 2015.
Effect on humans
The pollutants are absorbed through food and drinking water, through breathing the polluted air through the lungs (car exhaust fumes and tobacco smoke are the most important for the general population) and through the skin. The intake of harmful substances is particularly high in children.
PAHs degrease the skin, cause skin inflammation and can damage the cornea and irritate the respiratory tract, eyes and digestive tract.
Some PAHs are clearly carcinogenic in humans (e.g. lung, larynx and skin cancer as well as gastric and intestinal cancer or bladder cancer). There is a possibility of fruit damage or impairment of fertility. Thus, benzo [ a ] pyrene blamed for skin cancer in chimney sweeps.
Biomonitoring
The determination of 1-hydroxypyrene in urine is currently the method of choice for assessing exposure to polycyclic aromatic hydrocarbons.
Recognized occupational disease
Since August 2017, diseases such as changes in the mucous membrane, cancer or other neoplasms of the urinary tract caused by polycyclic aromatic hydrocarbons can be recognized as an occupational disease in Germany upon application, provided that they have been exposed to a cumulative dose of at least 80 benzo (a) pyrene years . This also applies to diseases that occurred before this date.
Verification procedure
Reliable identification and quantification takes place with the help of GC-MS coupling after adequate sample preparation . The results of available rapid tests with PAH indicator strips should be backed up with the methods mentioned above in order to prevent misinterpretations and to reliably assess the health and economic consequences.
literature
- Karsten Strey: The world of polycyclic aromatics ; Lehmanns Media, Berlin 2007, ISBN 978-3-86541-184-6 .
- Maximilian Zander : Polycyclic aromatics - hydrocarbons and fullerenes. Teubner Verlag, 1995, ISBN 3-519-03537-5 .
- Michael Herrenbauer: Biosorption of polycyclic aromatic hydrocarbons (PAH) on microorganisms and liposomes . Shaker Verlag , 2002, ISBN 3-8265-9903-9 .
- Tilman Gocht, Peter Gratwohl: Polycyclic aromatic hydrocarbons from diffuse sources. Atmospheric deposition and accumulation in rural soils. In: Environmental Sciences and Pollutant Research - Journal for Environmental Chemistry and Ecotoxicology. 16 (4), 2004, pp. 245-254; doi: 10.1007 / BF03039576 .
- Ronald G. Harvey: Polycyclic Aromatic Hydrocarbons. Wiley-VCH, 1997, ISBN 0-471-18608-2 .
- Ronald G. Harvey: Polycyclic Aromatic Hydrocarbons - Chemistry and carcinogenicity. Cambridge University Press, 1991, ISBN 0-521-36458-2 .
- C. Glende: Synthesis and mutagenicity studies of derivatives of pyrene, 1-nitropyrene and 1-aminopyrene. Cuvillier Verlag, Göttingen 2001, ISBN 3-89873-327-0 .
- Andreas Luch: The Carcinogenic Effects of Polycyclic Aromatic Hydrocarbons. Imperial College Press, 2005, ISBN 1-86094-417-5 .
- Wolfgang Mücke (Ed.): Analysis and mutagenicity of traffic-related fine dust: PAK and Nitro-PAK . Herbert Utz Verlag, Munich 2009, ISBN 978-3-8316-0941-3 .
- MT Wu, TC Lee et al .: Whole Genome Expression in Peripheral-Blood Samples of Workers Professionally Exposed to Polycyclic Aromatic Hydrocarbons. In: Chemical Research in Toxicology . 24 (10), 2011, pp. 1636-1643, doi: 10.1021 / tx200181q . PMID 21854004 .
Web links
- Polycyclic Aromatic Hydrocarbons (PAH) [MAK Value Documentation in German language, 2008] . In: The MAK Collection for Occupational Health and Safety . Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany January 31, 2012, p. 1–210 , doi : 10.1002 / 3527600418.mb0223orgd0045 .
- Stiftung Warentest: FAQ PAK: What you need to know about these pollutants from February 5, 2018
- mtm ingenieurgemeinschaft: Instructions for the evaluation and measures to reduce the PAH exposure through parquet floors with tar adhesives in buildings
- arguk.de: PAK in the interior ( Memento from September 6, 2012 in the Internet Archive )
- BfR : Polycyclic aromatic hydrocarbons (PAHs) in toys (PDF; 138 kB), updated BfR opinion No. 051/2009 of 14 October 2009
- Federal Office of Public Health : Polycyclic Aromatic Hydrocarbons (PAH)
Individual evidence
- ↑ Milan Randić, Xiaofeng Guo: Giant benzenoid hydrocarbons. Superphenalene resonance energy. In: New J. Chem. 23, 1999, pp. 251-260. doi: 10.1039 / A808949C
- ↑ Entry on polycyclic aromatic hydrocarbons. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ Claudia Synowietz (Ed.): Paperback for chemists and physicists . founded by Jean d'Ans, Ellen Lax. 4th edition. Volume II: Organic Compounds . Springer, Berlin 1983, ISBN 3-540-12263-X .
- ↑ a b c Entry on anthanthrene in the GESTIS substance database of the IFA , accessed on June 24, 2019 (JavaScript required)
- ^ PAH IR Spectral Database. In: astrochem.org. Retrieved October 4, 2010 .
- ↑ AGGM Tielens : Interstellar Molecules polycyclic aromatic hydrocarbon. In: Annual Review of Astronomy and Astrophysics . 46, 2008, pp. 289-337; doi: 10.1146 / annurev.astro.46.060407.145211 .
- ↑ A. Leger, JL Puget: Identification of the 'unidentified' IR emission features of interstellar dust? In: Astronomy and Astrophysics . 137, 1984, L5; bibcode : 1984A & A ... 137L ... 5L ; PDF .
- ↑ Article 67 of Regulation (EC) No. 1907/2006 in its Annex XVII under entry 31 for the substances listed in column 1.
- ↑ Jian Yan, Lei Wang, Peter P. Fu, Hongtao Yu: Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list. In: Mutation Research / Genetic Toxicology and Environmental Mutagenesis. 557 (1), 2004, pp. 99-108. PMC 2713671 (free full text).
- ↑ Risk factor PAH: Concentration in products alarmingly high. Press release TÜV Rheinland AG from March 31, 2009, March 6, 2018 available
- ↑ Suitcase: Poison under control . In: test.de , as of May 24, 2012, available on March 6, 2018.
- ↑ ÖKO-TEST: Test handbags and artificial leather, April 2016 , March 6, 2018 available
- ^ Stiftung Warentest: Basic information about PAK , February 5, 2018, accessed on April 11, 2018.
- ↑ a b Stiftung Warentest: Test of Plush Toys , November 26, 2015, accessed on April 11, 2018.
- ↑ Stiftung Warentest: Pollutants in everyday objects: What stinks is often dangerous , June 28, 2017, accessed on April 11, 2018.
- ↑ Black tea in the test: Darjeeling and Ceylon-Assam polluted . In: test.de , October 23, 2014, available on March 6, 2018.
- ↑ a b Regulation (EC) No. 1907/2006 Article 67 with Annex XVII, entry number 50, column 2 no. 1–4 for tires, no. 5–7 for consumer goods. Retrieved March 5, 2020
- ↑ Federal Institute for Risk Assessment: Polycyclic Aromatic Hydrocarbons (PAH) in toys. (PDF; 138 kB) Updated BfR Opinion No. 051/2009 of October 14, 2009, Section 3.1.3 “Exposure”; Available March 6, 2018
- ↑ Freya Riechert, Marion Berger, Norbert Kersten: Biomonitoring during impregnation of wood with coal tar oils - 1-hydroxypyrene in the urine as a marker for internal exposure to polycyclic aromatic hydrocarbons (PAH) . In: Zbl Arbeitsmed. 61, 2011, pp. 4–12.
- ↑ D. Pigini, AM Cialdella, P. Faranda, G. Tranfo: Comparison between external and internal standard calibration in the validation of an analytical method for 1-hydroxypyrene in human urine by high-performance liquid chromatography / tandem mass spectrometry. In: Rapid Commun Mass Spectrom. 20 (6), 2006, pp. 1013-1018. PMID 16479558 .
- ↑ Number 1321 of Appendix 1 to the Occupational Diseases Ordinance - BKV
- ↑ § 6 Paragraph 1 BKV
- ↑ K. Ziegenhals, HJ Hübschmann, K. Speer, W. Jira: Fast-GC / HRMS to quantify the EU priority PAH. In: J Sep Sci . 31 (10), 2008, pp. 1779-1786. PMID 18461643 .
- ↑ Naydenova S, Veli A, Mustafa Z, Gonsalvesh L: Qualitative and quantitative determination of polycyclic aromatic hydrocarbons in fine particulate matter. , J Environ Sci Health A Tox Hazard Subst Environ Eng. 2019 Dec 17: 1-12, PMID 31847692