Condensation reaction
In chemistry, a condensation reaction is a reaction in which two molecules combine with each other with the elimination of water - alternatively also ammonia , carbon dioxide , hydrogen chloride , an alkanol or another low molecular weight substance. Condensation reactions are also possible intramolecularly and are often reversible.
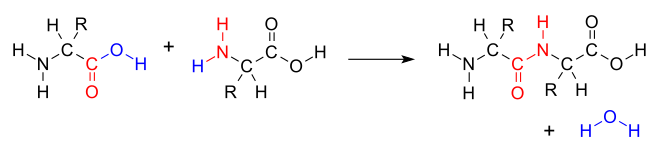
An example of the condensation reaction is the enzyme-catalyzed reaction of two amino acids to form a dipeptide according to the following reaction scheme:
Condensation plays an important role in organic chemistry . These include the following reactions:
- Aldol condensation
- Acyloin condensation
- Claisen condensation
- Dieckmann condensation
- Ether formation
- Nucleotide formation
- Peptide and Protein Formation
- Polycondensation for plastics production
- Ugi reaction
- Esterification
- Acid condensation
The condensation reaction is the basis for the production of many high molecular weight compounds, for example nylon , polyester and various epoxies , as well as for silicates and polyphosphates . The synthesis of biopolymers ( proteins , polysaccharides , fats , nucleic acids ) in the metabolism of the cells also takes place through condensation reactions.
The reaction in which monomers react to form a polymer is called the polycondensation reaction . In contrast to the other types of polymer production ( polymerization and polyaddition ), one or more by-products, the condensates (water, ammonia, alcohols or others), are released during the polycondensation. These by-products have to be removed continuously in order to obtain a high conversion ( law of mass action ). The reaction rate of a polycondensation must be well above 95%, since otherwise only short-chain oligomers are obtained ( Carothers equation ). In order for a monomer to be able to participate in the reaction, it must have at least two functional groups that are reactive (e.g. –OH, –COOH, –CO, –NH 2 , ...).
reversal
The reverse of the condensation reaction is hydrolysis (in the case of water; otherwise also aminolysis , alcoholysis , ...); mostly in an acidic environment.
Olation and Oxolation
In inorganic chemistry, olation is a bridging of elemental atoms with hydroxide ions (abbreviation: oil), which belongs to the group of condensation reactions, with the displacement of coordinatively bound water. Oxolation, on the other hand, is a bridging of elemental atoms with oxide ions (abbreviation: Oxo) with the elimination of water from the hydroxyl groups attached to the corresponding element. Olation and oxolation are responsible for many natural and synthetic materials, such as polymers and polyoxometalates .
literature
- KPC Vollhardt , NE Schore: Organic Chemistry. Wiley-VCH, Weinheim 2000, ISBN 3-527-29819-3 .
- R. Brückner: reaction mechanisms: organic reactions, stereochemistry, modern synthesis methods. 3rd, act. and revised Edition. Spectrum, 2004, ISBN 3-8274-1579-9 .
Individual evidence
- ^ Nils Wiberg, Arnold F. Holleman, Egon Wiberg , Gerd Fischer: Textbook of inorganic chemistry. 102nd, heavily redesigned. u. verb. Edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1159.
