Hexacene
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
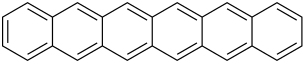
|
||||||||||
| General | ||||||||||
| Surname | Hexacene | |||||||||
| Molecular formula | C 26 H 16 | |||||||||
| Brief description |
dark green solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 328.41 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
380 ° C (decomposition) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Hexacene is a chemical compound from the group of acenes .
Appearance and properties
The first synthesis of hexacene was reported by Erich Clar in 1942 . In 1955 the compound was synthesized by the dehydrogenation of hexacosadehydrohexacene with palladium - carbon . In 1982 it was reported that hexacene is blue-green in color and decomposes at 380 ° C. In 2007, the first direct synthesis of hexacene based on a photochemical decarbonylation of a diketo precursor became known:
Hexacene is very reactive and can only be isolated in a polymethyl methacrylate matrix. It cannot be isolated in its pure form, as it dimerizes even at low concentrations and reacts in solution with oxygen to form organic peroxide. However, bis (trialkylsilyl) ethynylated derivatives of hexacene are more stable and can be isolated as crystalline solids.
Individual evidence
- ^ Manfred Hesse, Herbert Meier, Bernd Zeeh: Spectroscopic methods in organic chemistry , p. 17; ISBN 978-3-13-576107-7 .
- ↑ John Dalton Wright: Molecular crystals . Cambridge University Press, 1994, ISBN 978-0-521-47730-7 ( page 16 in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ E. Clar: A new synthesis of hexacene (Aromatic hydrocarbons, XXXIV. Communication) . In: Reports of the German Chemical Society . 75, No. 11, 1942, pp. 1283-1287. doi : 10.1002 / cber.19420751102 .
- ^ William J. Bailey, Chien-Wei Liao: Cyclic Dienes. XI. New Syntheses of Hexacene and Heptacene . In: J. Am. Chem. Soc. ; 1955 ; 77 (4); Pp. 992-993; doi : 10.1021 / ja01609a055 .
- ↑ H. Angliker, E. Rommel, J. Wirz: Electronic spectra of hexacene in solution (ground state, triplet state, dication and dianion) . In: Chemical Physics Letters . 87, No. 2, 1982, pp. 208-212. doi : 10.1016 / 0009-2614 (82) 83589-6 .
- ^ Rajib Mondal, Ravi M. Adhikari, Bipin K. Shah, Douglas C. Neckers: Revisiting the Stability of Hexacenes . In: Org. Lett. ; 2007 ; 9 (13); Pp. 2505-2508; doi : 10.1021 / ol0709376 .
- ^ John E. Anthony: The Larger Acenes: Versatile Organic Semiconductors . In: Angewandte Chemie International Edition . 47, 2008, p. 452. doi : 10.1002 / anie.200604045 .
