Polycycles
Polycyclic compounds or short polycycles (singular polycyclic ) are cyclic organic-chemical compounds in which the atoms are arranged in several rings. If the compounds contain only one ring, they are called monocycles instead . With very few exceptions, only carbon is able to form stable polycycles. If heteroatoms such as nitrogen , oxygen , sulfur or phosphorus are also present within the ring system , the compounds are called heterocyclic polycycles . If two or more rings are directly linked by single bonds, one speaks of ring sequences.
Polycycles are very common in nature. For example, steroids are polycycles.
nomenclature
Many polycycles have trivial names , which, however, are also allowed according to the IUPAC nomenclature. The systematic nomenclature of polycycles is sometimes complicated and depends on the number of rings, the total number of ring C-atoms ( parent hydrocarbons ), the number of atoms between the bridgehead C-atoms and the way in which the rings are linked are.
- For polycycles that consist of two rings, the designation bicyclic or bicyclic compound is common, e.g. E.g .: naphthalene . If there are three rings, one speaks of tricyclic compounds .
- Two directly adjacent rings with a common bond are also referred to as a condensed or fused ring system (examples: naphthalene , anthracene , phenanthrene , pyrene ).
| Spiro connections (selection) |
| Spiro [4.5] decane |
| 1,3,5-Triazaspiro [5.5] -undeca-1,3-diene, example of a heterocyclic spirane. |
| Dispiro [4.2.5.2] pentadecane, example of a dispirane. |
Differentiation according to the type of connection of the rings
- Spiro compounds are polycycles in which rings are linked by a single common atom. In chemical nomenclature, they are designated with the prefix Spiro- .
- Ring associations are ring systems that are linked to one another via single or double bonds. Example: biphenyl
- Condensed polycyclic compounds are polycycles in which the rings are each connected by exactly two common atoms (ie exactly one common bond). Examples: naphthalene , anthracene and phenanthrene . Aromatic condensed polycycles are also dealt with under the heading polycyclic aromatic hydrocarbons ( PAH ). Condensed systems that also have bridges are referred to as bridged condensed polycyclic systems.
- Bridged polycyclic compounds themselves are polycycles in which the rings are linked by two or more common atoms. In chemical nomenclature they are often referred to using the Von Baeyer system . Examples are the bicyclic terpenes Bornan (Camphan), Caran , Camphor , Fenchan , Fenchon , Pinan , the Pinene , and Thujon .
Differentiation according to missing or existing double bonds
Many polycycles can be formally traced back to an aromatic carbocyclic or heterocyclic backbone that contains the maximum number of double bonds according to the valence rules. If you add hydrogen atoms to these double bonds in your mind, in other words: "saturate" the double bonds , you get "hydro derivatives". In practice, this is often achieved through (catalytic) hydrogenation, but in principle the experiment does not play a role in the nomenclature.
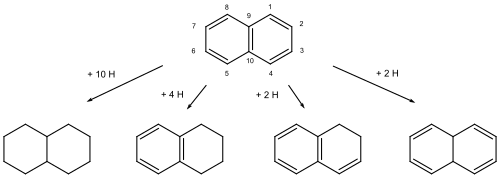
A typical example are the hydrogenated naphthalenes, such as the decalins . According to the Von Baeyer nomenclature , they are referred to as bicyclo [4.4.0] decane. However, the compounds were also obtained by hydrogenating naphthalene . A maximum of ten hydrogen atoms can be deposited without destroying the ring structure, which is why the hydrocarbon was called decahydronapthalene . Complete hydrogenation is also expressed in the nomenclature by the prefix “Perhydro-” . Formally and often in reality, partial (“partial”) hydrogenations of hydrocarbons with the maximum number of double bonds are possible. These “partially hydrogenated” compounds are characterized by the prefixes “Dihydro-” , “Tetrahydro-” , “Hexahydro-” and so on. The position of the “hydro-hydrogen atoms” is indicated by the numbering, the locants of the maximally unsaturated ring systems; in this way, isomers can optionally be distinguished.
Hydronaphthalenes: decahydronaphthalene (decalin), 1,2,3,4-tetrahydronaphthalene (tetralin), 1,2-dihydronaphthalene, 9,10-dihydronaphthalene. The hydrogen atoms are not shown. The first and last hydronaphthalene can have stereoisomers ( cis and trans ).
The formulas of bridged polycyclic hydrocarbons can be derived from the structural formulas of mono- or bicyclic hydrocarbons by linking two carbon atoms or inserting carbon chains ("bridges") (see article Bridged hydrocarbons ). A selection is shown in the table.
| Structural formula | Common name | systematic name |
|---|---|---|

|
Dewar benzene | Bicyclo [2.2.0] hexa-2,5-diene |

|
Benzvalen | Tricyclo [3.1.0.0 2,6 ] hex-3-ene |

|
Tetrahedrane | Tricyclo [1.1.0.0 2,4 ] butane |

|
Adamantane | Tricyclo [3.3.1.1 3.7 ] decane |

|
Twistan | Tricyclo [4.4.0.0 3.8 ] decane |

|
Prism | Tetracyclo [2.2.0.0 2.6 .0 3.5 ] hexane |

|
Trinorbornane | Tetracyclo [5.2.2.0 1.6 .0 4.9 ] undecane |
|
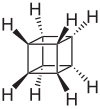
Cuban | Pentacyclo [4.2.0.0 2.5 .0 3.8 .0 4.7 ] octane |

|
Tetraasterane | Pentacyclo [6.4.0.0 2.7 .0 4.11 .0 5.10 ] dodecane |
|
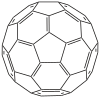
[60] fullerene |
Hentriacontacyclo [29.29.0.0 2.14 .0 3.12 .0 4.59 .0 5.10 .0 6.58 .0 7.55 .0 8.53 .0 9.21 .0 11.20 .0 13.18 .0 15.30 .0 16.28 .0 17.25 .0 19.24 .0 22.52 .0 23.50 .0 26.49 .0 27.47 .0 29.45 .0 32, 44 .0 33.60 .0 34.57 .0 35.43 .0 36.56 .0 37.41 .0 38.54 .0 39.51 .0 40.48 .0 42.46 ]
hexaconta-1 , 3.5 (10), 6.8,11,13 (18), 14,16,19,21,23,25,27,29 (45), 30.32 (44), 33.35 (43 ), 36.38 (54), 39 (51), 40 (48), 41,46,49,52,55,57,59-triaconts |
See also
- Asterans
- Diamantan
- Bridged hydrocarbons
- Phane
- Catenanes
- Helicene
- Rotaxanes
- Clar rule (after Erich Clar )
Individual evidence
- ↑ Eberhard Breitmaier and Günther Jung: Organische Chemie , p. 98ff; ISBN 978-3135415055 .
literature
- International Union for Pure and Applied Chemistry ( IUPAC ): Rules for the nomenclature of organic chemistry, German edition (Hrsg. H. Grünewald), Vol. 1, Section A - Hydrocarbons , Verlag Chemie, Weinheim, 1975.
- Dieter Hellwinkel: The systematic nomenclature of organic chemistry: instructions for use , 5th edition, Springer, Berlin, Heidelberg u. a. O., 2006, ISBN 3-540-26411-6 .