Thujones
Thujones ( absinthol , tanacetone ) are colorless liquids and form a group of bicyclic monoterpenes - ketones with a menthol-like odor. As part of their essential oils , thujones are found in thuja , thyme , wormwood , tansy , rosemary , mugwort and real sage .
The structure was cleared up in 1900 by Friedrich Wilhelm Semmler .
Representative
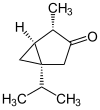
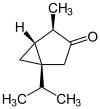
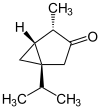
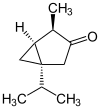
The thujones are the stereoisomers of 1-isopropyl-4-methylbicyclo [3.1.0] hexan-3-one.
| Thujones | ||||||||
| Surname | (+) - α-thujone | (-) - α-thujone | (+) - β-thujone | (-) - β-thujone | ||||
| Structural formula |
|
|
|
|
||||
| other names |
4-methyl-1- (propan-2-yl) bicyclo [3.1.0] hexan-3-one ( IUPAC ) |
|||||||
| CAS number | 546-80-5 | 471-15-8 | ||||||
| 1125-12-8 | ||||||||
| PubChem | 12304612 | 261491 | 91456 | 6553876 | ||||
| Molecular formula | C 10 H 16 O | |||||||
| Molar mass | 152.24 g mol −1 | |||||||
| Physical state | liquid | |||||||
| Brief description | light yellow liquid | |||||||
| boiling point | 201 ° C | |||||||
| density | 0.91 g cm −3 (20 ° C) | |||||||
| solubility |
very poorly |
|||||||
|
GHS labeling |
|
|
|
|
||||
| H and P phrases | see above | 302 | see above | see above | ||||
| see above | no EUH phrases | see above | see above | |||||
| see above | no P-phrases | see above | see above | |||||
| LD 50 | 500 mg kg −1 (rat, oral) | |||||||
Occurrence
In the wormwood plant ( Artemisia absinthium ) α- and β-thujones occur. In the production of absinthe , thujones are extracted from the leaves of wormwood ( Folia absinthii ) or the whole plant ( Herba absinthii ). Thujones are also found in many other Artemisias and z. B. also with a share of up to 60% in the essential oils of the real sage ( Salvia officinalis ) and also in the dried form of the white sage ( Salvia apiana ), which u. a. is used for smoking. Thuja oil contains 40% (-) - α-thujone, tansy oil 58% (+) - β-thujone.
Effects
Thujones are nerve toxins which, in higher doses , can cause confusion and epileptic cramps (convulsions). Other symptoms , such as B. dizziness , hallucinations and delusions that could be observed after ingestion of alcoholic beverages containing thujone were attributed to these active substances. These drinks, especially absinthe , are also advertised because of their alleged euphoric and aphrodisiac properties. Since the permissible thujone content in alcoholic beverages was limited to a maximum of 35 mg per kg and even in historical absinthe no higher values could be detected, the effect of absinthe consumption is now more attributed to alcohol. The symptoms of chronic absinthe consumption ( absinthism ) are identical to those of alcoholism . A study in 2008 only found concentrations averaging 25 mg / l in samples from the time before the ban. A psychotropic effect is extremely unlikely with these values.
GABA A receptors in particular are held responsible for the convulsive effects of thujones described . As antagonists and modulators of these receptors , thujones inhibit the anticonvulsant effect of γ-aminobutyric acid (GABA), albeit with a weak potency. As GABA A receptor antagonists, they have a similar effect to the plant toxins bicuculline of the heart flowers and the picrotoxin of the myrtle . A desensitization of 5-HT 3 receptors can also contribute to the observed effects. A possible common mechanism of action with the cannabis active ingredient tetrahydrocannabinol via an activation of cannabinoid receptors , which was suspected on the basis of distant analogies of the molecular structure and clinical effects, could not be confirmed. An activation of the taste receptor TAS2R14 is held responsible for the bitter taste of thujones .
Analytics
Reliable qualitative and quantitative determination in various test materials is possible after adequate sample preparation by coupling gas chromatography and / or HPLC with mass spectrometry . These methods are also used for metabolism studies .
Statutory Regulations
The aroma regulation limits the thujone content in food . Drinks and other foods may contain a maximum of 0.5 mg / kg. Food containing sage preparations may contain a maximum of 25 mg / kg thujone. The limit in alcoholic beverages (special regulation) depends on the alcohol content of the spirit :
Brandy:
- maximum 5 mg / kg with an alcohol content of up to 25% by volume
- maximum 10 mg / kg with an alcohol content of more than 25% by volume
Bitter spirits:
- maximum 35 mg / kg with an alcohol content of more than 25% by volume
Outside of foodstuffs, thujone falls under the definition of Section 2 (1) of the AMG as soon as it is intended for use on humans or animals. The production and sale of a substance is therefore regulated by the AMG, regardless of the form in which the substance is present, if it complies with Section 2 (1). The sale and manufacture of pharmaceuticals without a license is punishable under AMG § 2 Paragraph 1 No. 5 a. F., § 2 Abs. 1 Nr. 2a new F., § 5, § 95 Abs. 1 Nr. 1. This was confirmed in a judgment of the Federal Court of Justice on the freely available chemical γ-butyrolactone ( GBL ), which according to is classified as a medicinal product by the AMG as soon as it is intended for consumption or use on humans or animals.
The Tobacco Ordinance prohibits the addition of thujone to tobacco products.
The following limit values for thujone apply in the European Union:
- 0.5 mg / kg in foods with Artemisia sp. , with the exception of Salvia officinalis (sage) or non-alcoholic beverages
- 10 mg / kg in other alcoholic beverages
- 25 mg / kg in foods with Salvia officinalis
- 35 mg / kg in alcoholic beverages with Artemisia sp.
In the United States, adding thujone to food is not permitted. Food and beverages containing Artemisia sp. White cedar , oak moss , tansy , or common yarrow must contain less than 10 mg / l thujone. Other plants with thujone are not regulated, e.g. B. Sage and sage oil (with up to 50% thujone) are classified as Generally Recognized As Safe (GRAS, harmless substance).
In Canada, alcoholic beverages are regulated by the provinces. In Alberta, Ontario, British Columbia and Nova Scotia up to 10 mg / kg of thujone is allowed, in Quebec 15 mg / kg, in Manitoba 6–8 mg / L.
Individual evidence
- ↑ a b c data sheet (-) - α-Thujone from Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 85th edition. (Internet version: 2005), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-532.
- ↑ a b Erowid
- ↑ Data sheet α- and β-thujone (mixture of isomers) (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ Entry on Thujone. In: Römpp Online . Georg Thieme Verlag, accessed on April 29, 2014.
- ↑ RW Olsen: Absinthe and γ-aminobutyric acid receptors. In: Proc. Natl. Acad. Sci. USA Vol 97, 2000, pp. 4417-4418.
- ↑ SA Padosch, DW Lachenmeier, LU Kröner: Absinthism: a fictitious 19th century syndrome with present impact. In: Subst. Abuse Treat. Prev. Policy. Volume 1, 2006, p. 14.
- ↑ D. Lachenmeier, D. Nathan-Maister, T. Breaux, E.-M. Sohnius, K. Schoeberl, T. Kuballa: Chemical Composition of Vintage Preban Absinthe with Special Reference to Thujone, Fenchone, Pinocamphone, Methanol, Copper, and Antimony Concentrations. In: J. Agric. Food Chem. 56 (9), 2008, pp. 3073-3081.
- ↑ KM Hold, NS Sirisoma, T. Ikeda, T. Narahashi, JE Casida: α-thujone (the active component of absinthe): γ-aminobutyric acid type A receptor modulation and metabolic detoxification. In: Proc. Natl. Acad. Sci. USA Volume 97, 2000, pp. 3826-3831.
- ↑ T. Deiml, R. Haseneder, W. Zieglgänsberger, G. Rammes, B. Eisensamer, R. Rupprecht, G. Hapfelmeier: α-Thujone reduces 5-HT3 receptor activity by an effect on the agonist-reduced desensitization. In: Neuropharmacology . Volume 46, 2004, pp. 192-201.
- ↑ J. del Castillo, M. Anderson, GM Rubottom: Marijuana, absinthe and the central nervous system. In: Nature. Volume 253, 1975, pp. 365-366.
- ↑ JP Meschler, AC Howlett: Thujone exhibits low affinity for cannabinoid receptors but fails to evoke cannabimimetic responses. In: Pharmacol Biochem Behav . Vol. 62, 1999, pp. 473-480.
- ↑ M. Behrens, A. Brockhoff, C. Kuhn, B. Bufe, M. Winnig, W. Meyerhof: The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. In: Biochem. Biophys. Res. Commun. Volume 319, 2004, pp. 479-485. PMID 15178431 .
- ↑ B. Bach, M. Cleroux, M. Saillen, P. Schönenberger, S. Burgos, J. Ducruet, A. Vallat: A new chemical tool for absinthe producers, quantification of α / β-thujone and the bitter components in Artemisia absinthium. In: Food Chem. 213, Dec 15, 2016, pp. 813–817. PMID 27451252
- ↑ JD Williams, JA Yazarians, CC Almeyda, KA Anderson, GR Boyce: Detection of the Previously Unobserved Stereoisomers of Thujone in the Essential Oil and Consumable Products of Sage (Salvia officinalis L.) Using Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. In: J Agric Food Chem. 64 (21), Jun 1, 2016, pp. 4319-4326. PMID 27181395
- ↑ H. Hu, X. Zheng, H. Hu, R. Wang, Y. Wu: Study on gas chromatography-mass spectrometry fingerprint of Acanthopanax brachypus. In: J Chromatogr Sci. 52 (8), Sep 2014, pp. 759-765. PMID 24076562
- ↑ K. Abass, P. Reponen, S. Mattila, O. Pelkonen: Metabolism of α-thujone in human hepatic preparations in vitro. In: Xenobiotica. 41 (2), Feb 2011, pp. 101-111. PMID 21087116
- ↑ Flavor Ordinance (PDF; 33 kB)
- ^ Erwin Deutsch, Rudolf Ratzel, Hans-Dieter Lippert: Commentary on the Medicines Act (AMG). 3. Edition. Gabler Wissenschaftsverlage, 2010, ISBN 978-3-642-01454-3 , pp. 64–66 ( limited preview in Google book search).
- ↑ ArzneimittelG, Section 2, Paragraph 1, No. 5 a. F., Section 2 Paragraph 1 No. 2a new version, Section 5, Section 95 Paragraph 1 No. 1. Accessed on May 16, 2012.
- ↑ Martin Kämpf: Criminal Law: Trading with gamma-butyrolactone (GBL, liquid ecstasy) for consumption purposes. July 25, 2011.
- ↑ The unauthorized placing on the market of gamma-butyrolactone (GBL) for consumption purposes is punishable under the Medicines Act. BGH judgment of December 8, 2009, 1 StR 277/09, LG Nürnberg-Fürth at Lexetius.com/2009,3836.
- ↑ Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 2008 (PDF) , European Commission .
- ^ Opinion of the Scientific Committee on Food on Thujone Scientific Committee on Food (2003) Accessed October 28, 2006.
- ↑ Laurie C. Dolan, Ray A. Matulka, George A. Burdock: Naturally Occurring Food Toxins . In: Toxins . tape 2 , no. 9 , 2010, p. 2289-2332 , doi : 10.3390 / toxins2092289 , PMID 22069686 , PMC 3153292 (free full text).
- ↑ FDA Regulation 21 CFR 172.510 - Food Additives Permitted for Direct Addition to Food for Human Consumption. Food and Drug Administration (2003). Retrieved October 28, 2006.
- ↑ Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau Industry Circular 2007-5 ( Memento of the original dated February 9, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved October 17, 2007. Retrieved May 5, 2009.
- ↑ Substances generally recognized as safe. ( Memento of the original from November 30, 2005 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Food and Drug Administration (2003). Retrieved October 28, 2006.


