Essential oils
| Structural formulas of some compounds from essential oils (without stereochemistry) |
|---|
 α-terpinene |
 menthol |
 Citronellal |
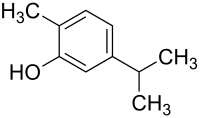 Carvacrol |
 Dillapiol |
Essential oils (also essential oils) are highly volatile and often highly flammable mixtures of substances, which consist of various organic substances that are soluble in one another, such as alcohols , esters , ketones or terpenes . They are obtained synthetically or from natural sources through steam distillation , extraction or pressing of the plants or parts of plants. Essential oils are often produced in the leaves of plants and stored in the plant tissue. The plants attract insects or repel pests.
In contrast to fatty oils, essential oils evaporate without leaving any residue.
ingredients
Essential oils mostly consist of mixtures of different terpenes , sesquiterpenes or aromatic compounds (e.g. phenylpropane derivatives). Terpenes are formally derived from isoprene units . Monoterpenes consist of two, sesquiterpenes of three isoprene units. The table below shows some examples of the various molecules found in essential oils. Residues of fat-soluble pesticides in the starting material can accumulate in the likewise fat-soluble essential oil.
| Substance group | acyclic | monocyclic | bicyclic | |
|---|---|---|---|---|
| Monoterpenes | Hydrocarbons | Ocimen , myrcene | Limonene , α-terpinene , phellandrene | α-pinene , camphene |
| Alcohols | Linalool , geraniol | menthol | Sabinol , Borneol | |
| Aldehydes | Neral , citronellal | |||
| Ketones | Carvone , menthone | Camphor , fenchone | ||
| Ether | Menthofuran , cineole , anethofuran | |||
| Ester | Geranyl acetate , linalyl acetate | Bornyl acetate , isobornyl acetate | ||
| Sesquiterpenes | Farnesol , Farnese | α-bisabolol , α-caryophyllene | Chamazulene , β-caryophyllene | |
| Substance group | Examples | |||
| Aromatics | Phenols | Carveol , carvacrol , thymol | ||
| Phenylpropanoids | Apiol , cinnamaldehyde , anethole , dillapiol , estragole | |||
| Furano coumarine | Coriandrin | |||
properties
Essential oils contain phytochemicals that can be used to attract insects to pollinate, to keep pests away or to protect against diseases (e.g. caused by bacteria or fungi).
Essential oils are made up of many different chemical compounds. They are fat-soluble , but do not contain fats. In contrast to fatty oils , essential oils evaporate without leaving any residue. As a rule, they are hydrophobic and insoluble or only slightly soluble in water. At normal pressure their boiling point is well above that of water, but they are distilled over by superheated steam. They usually have a lower density than water and therefore form droplets of liquid floating on the surface of the water (an exception is e.g. cinnamon oil ).
education
Essential oils are formed in the oil glands of plants and stored in plant tissue. They can be found in flowers, leaves, seeds, fruit peels, roots, resins, bark or in wood. Some plants provide essential oils from different parts of the plant that differ greatly in their chemical composition, e.g. B. cinnamon bark and cinnamon leaf oil.
Extraction
The most common method for obtaining essential oils is steam distillation . For this purpose, water vapor is blown into a closed kettle with crushed plant material. The water vapor drives the essential oil out of the plant. The oil-water mixture condenses in a cooled pipe and is then led into a collecting container. There the substances in the oil are separated from the aqueous phase and can be separated. The yield in relation to the starting material is usually in the one to two-digit per mil range. Some plants that cannot be distilled on their own, such as B. algae, nettle or hay can be distilled together with another plant as a carrier by means of co-distillation . Oils of some types of flowers, such as jasmine , tuberose or mimosa , cannot be obtained by steam distillation.
The cold pressing is only used for citrus oils. The peels are pressed to create an emulsion of liquid and essential oil. The oil is separated by centrifugation .
Extraction is mainly practiced with flower oils. For this purpose, all soluble aromatic substances (as well as waxes and colorings ) are removed from the plants by a solvent such as hexane , Florasol or supercritical carbon dioxide . The solvent is then distilled off. What remains is a waxy mass that is extracted again with alcohol or distilled. Such essential oils are also called absolutes . A residue control can ensure that there is no longer any solvent in the essential oil. The very expensive extraction with fats, the so-called enfleurage , is hardly practiced today.
processing
Most essential oils are used unchanged. Some, however, are first concentrated or further broken down, for example by distillation or adsorption. In this way, components of the essential oil that are desired for the effect can be concentrated, while unsuitable components can be removed. Mixtures that contain only one or a few main components can be obtained via distillation or crystallization , as is the case with the production of eugenol from clove oil. The importance of the extraction of individual components of natural essential oils has decreased significantly with the development of synthetic manufacturing processes.
history
The Egyptians were already familiar with distillation processes and used cedar oil , as well as the aromas of various plants. The Greeks probably adopted the art of distillation from the Egyptians; some distillation processes have already been described in Dioskurides . The Romans also liked to use perfume and incense , but did not develop the art of distillation any further. It is not known whether the perfumes in particular were essential or flavored fatty oils. In connection with alcohol distillation, the art of distillation revived among the Arabs from the 9th century . The main focus of the distillation books up to the 16th century was on "distilled wines" and "distilled waters". In the 17th century, the semi-industrial production of essential oils began, which was initially used for perfume rather than pharmaceuticals. Steam distillation has been used in industry and laboratories since 1826, from which distillation with pressurized steam, which is still common today, developed. The development of manufacturing and analysis methods can be traced back to the pharmacopoeia literature. In the Pharmacopoea Germanica (1872) only a short section was devoted to essential oils. It was only later, in DAB 5 (1910) and DAB 6 (1926), that the tests for essential oils were increased. The foundation stone for the development of modern analytical methods was laid by the structural elucidation of the constituents of essential oils by Otto Wallach (1847–1931) and the synthetic production by Friedrich Wilhelm Semmler (1860–1931).
use
Essential oils are used differently depending on their properties. The use as a fragrance in the cosmetics and perfume industry is often in the foreground, but certain essential oils are also important as medicinal substances and as technical solvents.
They are used in the cosmetics industry and for home aromatization in fragrance lamps . They are also important as taste-enhancing ingredients in spices and other foods. Some oils produced on a large scale such as orange peel oil and turpentine oil are also used as technical solvents.
Medicine and naturopathy
Some over-the-counter medicines contain essential oils as active ingredients, such as: B. eucalyptus or menthol to dissolve mucus in catarrh of the upper respiratory tract, bronchitis, etc. Also effects against flatulence and cramps in the gastrointestinal area, z. B. through teas with fennel - caraway - aniseed , especially in pediatrics, and inflammations in the mouth and throat area ( sage , chamomile ), are attributed to essential oils.
Essential oils play a central role in the naturopathic method of aromatherapy , a form of herbal medicine used to treat sensory disorders and diseases caused by fragrances. In addition to use by therapists (usually non-medical practitioners ), self-treatment with fragrance lamps , bath additives, sauna infusions or teas is widespread, whereby the boundaries between healing treatment and pure living room aromatization are fluid.
Most essential oils are skin-irritating and are therefore only used very diluted, e.g. B. as a component of oil-based skin care products or in conjunction with vegetable oils. Allergies and intolerance to essential oils occur, as well as asthmatic attacks in people who are sensitive to individual substances (e.g. menthol ). Often, when there is an intolerance to certain plants, reactions to the corresponding essential oils also occur.
Effect on the human body
Essential oils enter the bloodstream and tissues relatively easily when they come into contact with the skin or when they are inhaled . After aromatherapy, the scents have an impact on feelings, the vegetative nervous system , hormone production or the immune system .
toxicology
Some essential oils have a high allergy potential. It affects not only artificial essential oils, but also natural ones in particular. According to a study by the Federal Environment Agency, there are at least 500,000 people who are allergic to fragrances in Germany. It is particularly problematic when natural essential oils such as geraniol , linalool or limonene evaporate as an aerosol and oxidize in the oxygen in the air. This creates oxidation products with a strong sensitizing effect, which can trigger asthma-like symptoms or damage the respiratory organs. The use of such oils in fragrance lamps or in household products such as hairsprays or creams is problematic because they are diffused in an apartment or bedroom by evaporation. It is not without reason that the EU Scientific Advisory Committee (SCCNFP) has published a list of 26 fragrances that have a sensitizing effect and are subject to declaration. The list is based on the investigations carried out by the Information Association of Dermatological Clinics (IVDK). There are also essential oils such as estragole with suspected carcinogenic potential.
Use in perfume
In contrast to fatty oils, essential oils do not leave any stains on textiles, which is due to the fact that they evaporate completely. For this reason, essential oils are often used in perfumes. Since most essential oils are very irritating to the skin and mucous membranes, they are diluted with water or alcohol. How high the concentration of the fragrance contained in a perfume is can already be seen from the name. The "Eau" in Eau de Parfum stands for water and thus indicates a diluted perfume. Products with the name Parfum have the highest concentration of essential oils (up to 40%), followed by the Eau de Parfum (up to 15%), the Eau de Toilette (up to 8%), which has the lowest concentration of essential oils the Eau de Cologne (up to 4%). Heavily diluted perfumes are also known as scented water.
Natural oils
The terms natural, natural, nature-identical and artificial are used to differentiate between the essential oils. Natural oils are obtained directly from plants. The oils are differentiated according to their origin, properties, process and product quality. The description of a natural oil can include:
- the way the plant is grown (conventional, organic (kba) or wild collection)
- the German and botanical names of the plant
- the country of production of the plant
- the plant part used ( root , flower , fruit or leaf )
- the chemotype of the plant (in plants of a species that only differ in their chemical composition). In some plants, these can cause differences in the composition of the oil. Depending on the chemotype of the thyme, for example, thymol or linalool is included.
- Method of extraction of the essential oil
- In the case of extraction: information on the solvent and, if necessary, information on residue controls
- For viscous essential oils (e.g. vanilla, tonka) the type of diluent (e.g. alcohol, jojoba oil ) and the mixing ratio
- Information on biochemical and physical analyzes (partly available from the manufacturer according to batch numbers)
Natural oils
Natural oils consist of several naturally pure components, so they are not obtained exclusively from the plant that gives it its name. Natural oils must not contain any synthetic additives. A mixture of a natural oil with synthetic additives is called natural / nature-identical (N / NI).
Nature-identical oils
The components of nature-identical oils are produced synthetically based on the chemical composition of natural essential oils, so that they smell similar to natural oils. The composition of nature-identical oils is often less complex than that of the natural variants; nature-identical rosemary oil, for example, consists of around eleven components, while the natural essential oil has around 150 components.
Artificial oils
Artificial oils do not occur in nature. They are specifically designed for a specific smell.
durability
In contrast to the fatty oils, essential oils do not go rancid. However, if improperly stored, some essential oils can oxidize with the oxygen in the air, creating toxic reaction products. Reactions between the components in a perfume are also possible. In this way a perfume can "mature" like a good wine.
market
The global market for a total of around 120 different essential oils is estimated at over € 600 million; demand is relatively stable at 50,000 to 60,000 tons per year, around a third of which is citrus oils. More than 50% of the amount on the world market comes from China. Hardly any essential oils are produced in Germany, as very few of the original plants for the oils thrive at home. Because of the very energy-intensive distillation, the energy costs are another disadvantage of the location. Production in Germany is only economical for chamomile and lemon balm.
literature
- Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg: Flavors and Fragrances . In: Ullmann's Encyclopedia of Industrial Chemistry , 2005, Wiley-VCH, Weinheim, p. 84, doi : 10.1002 / 14356007.a11_141 .
- Eduard Gildemeister , Friedrich Hoffmann (Ed.): The essential oils. Berlin 1899 ff.
- Johanna Graßmann, Renate Spitzenberger, Susanne Hippeli, Renate Vollmann, Erich F. Elstner: Essential oils from mountain pine . In: Naturwissenschaftliche Rundschau . 58 (3), 2005, pp. 127-133, ISSN 0028-1050
- Karl-Heinz Kubeczka: The essential oils of different types of Ruta. In: Herba hung. Volume 10, No. 2-3, 1971, pp. 109-118.
- Wolfgang Legrum: Fragrances, between stench and fragrance , Springer, Wiesbaden 2015, ISBN 978-3-658-07309-1 .
- Bettina Malle, Helge Schmickl: Make your own essential oils . Verlag Die Werkstatt, Göttingen 2007, ISBN 978-3-89533-552-5 .
- D. Gary Young: Raindrop Technique . Essential Science, 2003, ISBN 978-0-943685-36-6 .
Web links
- Essential oils . ( Memento from April 12, 2010 in the Internet Archive ) Umweltlexikon-online.de
Individual evidence
- ↑ Both spellings are used in the specialist literature, Duden allows both spellings, source: http://www.duden.de/rechtschreibung/aetherisch_aetherhaltig
- ↑ Entry of essential oils and fragrances , in Seilnachts Chemielexikon, accessed on May 1, 2017 at: http://www.seilnacht.com/Chemie/problema.html#etherischeoele
- ↑ Thomas Seilnacht: Active ingredients of medicinal plants , accessed on May 3, 2017 at: http://www.digitalefolien.de/biologie/ Pflanzen / heilk / bioheil.htm
- ↑ Dietrich Frohne, Uwe Jensen: Systematics of the plant kingdom with special consideration of chemical characteristics and plant drugs . 4th edition, Gustav Fischer Verlag, Stuttgart / Jena / New York, ISBN 3-437-20486-6 , p. 311.
- ↑ Karl-Heinz Kubeczka: Comparative investigations on the biogenesis of volatile products of secondary metabolism, I .: Investigations on Ruta graveolens L. In: Flora, Abt. A. Volume 158, No. 5, 1967, pp. 519-544.
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 77-79 .
- ↑ Fr. Meyer, E. Meyer: Percutaneous absorption of essential oils and their ingredients. In: Drug Research. Volume 9, No. 8, 1959, pp. 516-519.
- ↑ Federal Environment Agency: Fragrances , accessed on May 3, 2017 at: http://www.umweltbundesamt.de/themen/gesundheit/umwelteinfluesse-auf-den-menschen/chemische-stoffe/duftstoffe .
- ↑ Entry of essential oils and fragrances , in Seilnachts Chemielexikon, accessed on May 1, 2017 at: http://www.seilnacht.com/Chemie/problema.html#etherischeoele .
- ↑ Legrum, Wolfgang: Fragrances, between stink and fragrance , pp. 160–162 Springer, Wiesbaden 2015, ISBN 978-3-658-07309-1 .
- ↑ Market Analysis of Renewable Raw Materials , Part II. (PDF) Agency for Renewable Raw Materials, meó Consulting Team, Fiber Institute Bremen, nova Institute, 2007, p. 343.


