Terpenes

The terpenes [ tɛʁˈpeːnə ] are a very heterogeneous and very large group of chemical compounds that occur naturally as secondary components in organisms. Formally, they are derived from isoprene and are characterized by a large variety of carbon skeletons and a smaller number of functional groups .
Over 8,000 terpenes and over 30,000 of the closely related terpenoids are known. Most terpenes are natural substances , i.e. mainly of vegetable and, more rarely, animal origin. In nature, predominantly hydrocarbon , alcohol , glycoside , ether , aldehyde , ketone , carboxylic acid and ester terpenes occur, but representatives of other groups of substances can also be found among the terpenes. The terpenes are the main component of the essential oils produced in plants .
Terpenes are often biological and have been of pharmacological interest for a long time ; however, their biological functions have only been poorly researched. They can be used as environmentally friendly insecticides by acting as pheromones to lure insects into traps. In addition, many have antimicrobial effects . Many terpenes are used as fragrances or flavorings in perfumes and cosmetic products.
Because of the large number and their diverse structural variants, there are several classification options for terpenes. The IUPAC, for example, only counts hydrocarbons among the terpenes, whereas all oxygen-containing isoprene derivatives are considered terpenoids. In practice, trivial names have become established for the carbon frameworks, which are often derived from the scientific name of the organism of the first isolation.
history

The terpenes were named after a suggestion by the French chemist Marcelin Berthelot by August Kekulé after the tree resin turpentine , which contains hydrocarbons as well as resin acids . Originally only these were called terpenes; the term was later expanded and specified more precisely. The most important researchers in the field of terpenes were, among others, Otto Wallach and Leopold Ružička . Both scientists were honored with the Nobel Prize in Chemistry (O. Wallach 1910 and L. Ruzicka 1939) for their work in researching terpenes .
In the early days of natural product isolation, in the 19th century, the elucidation of chemical structures was very complex, which led to the fact that many chemically identical terpenes were given different names, which were largely derived from their biological origin. It was not until 1884 that Otto Wallach showed that many of these connections were actually identical. In 1892 Wallach was able to clearly describe the first nine terpenes and published his findings in the book Terpenes und Camphor in 1914 . He recognized that the terpenes are based on isoprene units. Another pioneer in the field of structural elucidation of the terpenes was Adolf von Baeyer . Despite the research, very few lower terpenes were clearly identified for a long time. It was not until 1910 that the first correct formula for a sesquiterpen, the santalen , could be determined by Friedrich Wilhelm Semmler .
The biogenetic isoprene rule was drawn up by Otto Wallach in 1887 and formulated under this name by Leopold Ružička in 1922 . The biosynthesis of terpenes was finally clarified by Feodor Lynen and Konrad Bloch in 1964. Both scientists were honored with the Nobel Prize in Physiology or Medicine .
biosynthesis
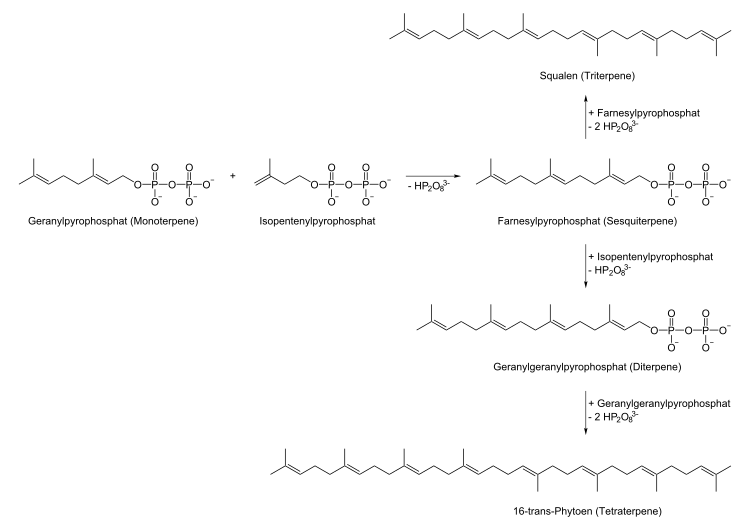
What the terpenes have in common is that they can be traced back to a skeleton whose basic unit is the unsaturated hydrocarbon isoprene , one or more times . A central building block in the biosynthesis of terpenes is dimethylallyl pyrophosphate (DMAPP) and its double bond isomer isopentenyl pyrophosphate (IPP). Both building blocks contain five carbon atoms and a double bond and can be understood as activated isoprene . The biosynthetic pathway presented by F. Lynen and K. Bloch starts from acetyl-CoA as the original building block, which is converted to mevalonic acid . The building blocks DMAPP and IPP are then synthesized from the mevalonic acid. An alternative biosynthetic pathway that has only recently been found, the so-called MEP (2C-methyl-D-erythritol-4P) pathway, is based on a sugar derivative as a C-5 building block. However, this path has so far only been observed in a few microorganisms, in green algae and plants. Both paths take place independently of each other. The mevalonate biosynthetic pathway is located in the cytosol and the MEP biosynthetic pathway in the plastids (chloroplast, leukoplast, etc.). The exchange between substrates of the two metabolic pathways is minimal. All mono- and diterpenes are synthesized in the plastids and all sesquiterpenes in the cytosol.
The further synthesis begins with DMAPP and IPP, the end products of the mevalonate route:
Farnesyl pyrophosphate synthase links one molecule of DMAPP and two molecules of IPP to form farnesyl pyrophosphate (FPP), which is the basic structure of the cytosolic sesquiterpenes. Squalene (squalene synthase), the basic structure of the triterpenes, is formed from two units of farnesyl phosphate. The plastid geranylgeranyl pyrophosphate synthase links one molecule of DMAPP with three molecules of IPP to form geranylgeranyl pyrophosphate (GGPP), the basic structure of the diterpenes. GGPP can in turn react to form 16- trans phytoene, the basic structure of the tetraterpenes. Each terpene is represented by the biosynthesis in that the key enzymes in terpene biosynthesis, the terpene synthases , convert these basic structures into the respective terpenes.
properties
Most terpenes are sparingly soluble in water, but dissolve well in non-polar solvents such as chloroform or diethyl ether . If necessary, however, they can also be brought into aqueous solution with solubilizers or in the form of liposomes .
Mono-, sesqui- and, to a limited extent, diterpenes are particularly volatile in steam, which is exploited when they are obtained from plants by steam distillation .
Terpenes have irritating (skin, respiratory tract) and allergizing potential.
Analytics
Today, all spectroscopic and spectrometric methods such as NMR spectroscopy (one and multi-dimensional) and mass spectrometry are used to determine the structure of terpenes . Terpene structures have also been clarified and corrected with the help of crystal structure analysis.
Gas chromatography is used to analyze known terpenes , often in combination with a mass spectrometer. Here the terpenes can be identified on the one hand by their retention times and the characteristic fragmentation pattern in the mass spectrum in comparison with a known reference.
Extraction

Terpenes, especially mono-, sesqui- and diterpenes, can be obtained from plants (parts of) or essential oils by physical methods such as steam distillation , extraction or chromatography . The juvenile plants typically supply the terpene hydrocarbons and the older plants increasingly the oxygen-containing derivatives such as alcohols , aldehydes and ketones .
There are chemical methods for the large-scale synthesis of terpenes, which are usually very specific.
Classification
The terpenes are lipids in the systematics of organic chemistry . The terpenoids are a subgroup of the terpenes ; here carbon atoms were discharged in later steps of the biosynthesis. Your carbon number is therefore no longer divisible by 5. Belonging to the terpenes is based on a common biosynthesis and the C 5 rule, not on common properties. The common building block of all terpenes is isoprene. The terpenes are among the secondary plant substances .
A general distinction is made between acyclic, mono-, bi-, tri-, tetra- and pentacyclic terpenes, i.e. molecules without, with one, with two, three, four or five rings. The terpenes also differ in the carbon structure on which they are built. In addition, they are classified according to their secondary substance group.
A distinction is also made as to whether the isoprene units are connected head-to-tail , head-to-head or tail-to-tail . This is called the "biogenetic isoprene rule". The side of the isoprene unit that contains the isopropyl group is called the head , the end of the isoprene unit that is unsubstituted is called the tail , in the sense of linking the units to form longer building blocks.
The terpenes are divided into isoprene units (always 5 carbon atoms each), which have the same number of carbon atoms. Terpenes with 5 carbon atoms are called hemiterpenes (C 5 ), with 10 monoterpenes (C 10 ), with 15 sesquiterpenes (C 15 ), with 20 diterpenes (C 20 ), with 25 sesterterpenes (C 25 ), with 30 triterpenes (C 30 ) and with 40 tetraterpenes (C 40 ). Terpenes with more than 8 isoprene units, i.e. with more than 40 carbon atoms, are called polyterpenes (greater than C 40 ). The names come from the Greek or Latin numerals: hemi = one half, mono = one, sesqui = one and a half, di = two, ... The isoprene unit is counted as half a terpene.
Hemiterpenes
The hemiterpenes are based on an isoprene unit. About two dozen hemiterpenes are known, which are extremely rare in nature in unbound form. The most important are the hemiterpenes Prenol and carboxylic acids tiglic , angelic acid , senecioic and isovaleric . However, they often occur as pyrophosphates as a biosynthesis intermediate of the terpenes and are glycosidically bound.
Monoterpenes


The monoterpenes are based on two isoprene units, i.e. a basic structure with 10 carbon atoms. Over 900 monoterpenes are known. All are synthesized from geranyl pyrophosphate by monoterpene synthases ; this takes place via a series of complex organic-chemical reactions that lead to the great structural diversity of the monoterpenes. Monoterpenes have a high bioavailability for hydrocarbons and an anti-carcinogenic effect has been found in animal experiments.
Tricyclic monoterpenes are extremely rare, an example being tricyclic .
Monoterpenes, along with sesquiterpenes, are main components of essential oils that are produced in large quantities by plants. For example, up to one liter of monoterpenes can be obtained from one square meter of forest soil covered with needle litter. Monoterpenes can be found in the components of over 2,000 plants from 60 different families.
Acyclic monoterpenes

Important acyclic hydrocarbon monoterpenes are myrcene , ocimene and Cosmen . All are constituents of essential oils. Linalool is found in roses - and up to 50 percent in lavender oil . Coriander and palmarosa oil contain geraniol and nerol . Citronellol can be obtained from citronella oil, Myrcenol from thyme oil . Also Lavandulol found in lavender oil. Ipsdienol is a fragrance in the flowers of orchid species . These compounds are common acyclic monoterpene alcohols.
The terpene aldehydes neral and geranial form the stereoisomer mixture citral , which smells intensely of lemon and is therefore used in aromas. Citronellal is used as an insect repellent. Geranic acid is a monoterpene carboxylic acid .
As furanoid acyclic monoterpenes include perillene and Rosenfuran to name. Rosenfuran is an odor-determining component of rose oil. Perill is found in essential oils and is a defense pheromone.
Monocyclic monoterpenes

Most of the monocyclic monoterpenes that can be derived from p- menthane have a cyclohexane structure . Also the grapefruit mercaptan , the stuff with the smallest known odor threshold , easily categorized here. But there are also those with cyclopentane ring as Junionon , or cyclobutane skeleton such as grandisol , a pheromone of the boll weevil , are known for the various synthetic routes, including photochemical processes. The monoterpenes containing a cyclopropane framework include chrysanthemol and chrysanthemum acid , the esters of which include some pyrethrins .
The monocyclic monoterpenes with a cyclohexane structure are mostly subdivided according to their secondary group of substances. The most important hydrocarbons here are menthan , limonene , phellandrene , terpinolene , terpinene and p- cymene . Compared to the other monoterpene hydrocarbons, menthane is rather rare in nature. Limonene occurs very frequently in a wide variety of plants, terpinolene and terpinene are fragrances and components of essential oils, terpinolene is an alarm pheromone of termites . Phellandren is found in caraway , fennel, and eucalyptus oils . p -Cymol is found in common savory .
Menthol is the main component of peppermint oil , it is an analgesic and is used for other medicinal applications. Also pulegol found in peppermint oils. Piperitol is found in species of eucalyptus and peppermint. Terpineol is a fragrance. Carveol is found in citrus oils. Thymol is found in the essential oils of thyme and oregano. Dihydrocarveol is found in cumin , pepper , celery and mint . Anethole is found in anise and fennel .
Menthon and pulegon , as well as their isomers, occur like menthol in peppermint oils. Phellandral is found in water fennel oil. Carvone and carvenon are found in caraway and dill, piperitone in eucalyptus oils .
1,4-Cineol and 1,8-Cineol are bicylic terpenes bridged by an ether bridge. 1,8-cineole has a bactericidal effect and is mainly found in eucalyptus and laurel and together with 1,4-cineole in juniper. Ascaridol , a peroxide , is found in goosefoot species.
Rose oxide and nerol oxide are fragrances in rose oil.
There are around 200 monoterpenes with a cyclopentane skeleton. They are divided into the iridoids and secoiridoids . The compounds were first discovered in a species of ant ( Iridomyrmex ) and are therefore some of the few terpenes of non-plant origin. They are characterized by a basic structure that contains a six-membered ring and a five-membered ring (cyclopentane pyrane structure). The release of carbon molecules from the basic structure creates terpenoids that no longer belong to the terpenes. The iridoids include, for example, aucubin and catalpol from ribwort ( Plantago lanceolata ) and loganin from bitter clover. In valerian ( Valeriana officinalis ) and devil's claw ( Harpagophytum procumbens ) are iridoid glycosides iridoids and contain.
Bicyclic monoterpenes
The bicycles Caran, Thujan, Pinan , Bornan (also outdated: Camphan ) and Fenchan, but also isobornylan and isocamphan, are the most important parent compounds of the bicyclic monoterpenes.
3-Carene is found in turpentine oils (in Russian turpentine oil, from Pinus sylvestris , as the second most common component), the oil of black pepper and is also found in citrus oils, firs and juniper species. Thuja is found in coriander and dill and, in addition to sabines, in oils. Thujone occurs in wormwood , which is processed into absinthe and wormwood , for example . Thujanol comes in the tree of life - juniper - and Artemisia TYPES ago. Carboxylic acids or carane Care framework, such as the Chaminsäure come in as cypresses ago.
2- pinene (α-pinene) is the main component of turpentine oils, 2 (10) -pinene (β-pinene) is also often found. Verbenol is a component of turpentine , together with the verbenone found in the oils of rosemary, it is a sexual pheromone of the bark beetle . Pinocarvone is found in eucalyptus oils and is sex pheromones of the pine moth ( Bupalus piniaria L.).
Camphor stimulates blood circulation, dissolves expectoration and has many other medicinal properties. It can be isolated from the camphor tree, as can borneol . Isoborneol is found in many composites .
The fenchans , especially fenchone and fenchol and their derivatives, occur in several essential oils. Fenchen and its derivatives are rare in essential oils.
Anethofuran is a bicyclic monoterpene with a furan body .
Sesquiterpenes
The sesquiterpenes are based on three isoprene units, i.e. a basic structure with 15 carbon atoms (and thus one and a half - in Latin sesqui - times as many as the monoterpenes made up of two isoprene units). There are more than 3,000 sesquiterpenes, making them the largest subgroup of terpenes. They are derived from farnesyl pyrophosphate . Around twenty of the numerous sesquiterpenes are of economic importance as fragrances and aromas.
Acyclic
Farnesyl pyrophosphate , the parent compound of sesquiterpenes, is found in oil shale , for example , and farnesol in rose and jasmine oil. Nerolidol is found in orange blossoms, Sinensal in orange oil. Furanoid acyclic sesquiterpenes Dendrolasin , Sesquirosenfuran and Longifolin . Dendrolasin is not only of vegetable origin, it is also found in ants (the name comes from the ant genus Dendrolasius ). The abscisic acid regulates the growth of plants; it is not synthesized directly from farnesyl pyrophosphate, but via the detour of the carotenoid metabolism (see tetraterpenes).
Monocyclic
The monocyclic sesquiterpenes are mainly divided into the parent compounds Bisabolane, Germacrane , Elemane and Humulane .
Over 100 bisabolans occur naturally in plants. Zingiberen is found in ginger oil. β-bisabolene is found in false cypresses and pine species, the anti-inflammatory bisabolol in chamomile oil. Sesquisabinen occurs in black pepper, sesquithujen in ginger. Derived from Germacran Periplanone are sex pheromones. Bicycloelemen and Elemol are derived from Eleman, Elemol is found in citronella oil, Bicycloelemen in peppermint oil. Many terpenes derived from humulan are found in the oil of hops .
Polycyclic
Most of the sesquiterpenes are polycyclic. Of the almost 30 caryophyllene , the most important one is caryophyllene ; it is found in caraway, pepper and cloves. About 450 of the Eudesman and Furanoeudesman are known. Selinene is found in celery and cannabis, eudesmol in eucalyptus species, costol in costus root oil. The santonins have an anti-helmint effect . Tubipofuran is an important furanoeudesman . The approximately 150 known eremophilans and valerans are found mainly in higher plants. Nootkatone and 11-eremophil-2,9-dione are flavorings in grapefruit oil. Of the Cadinanen about 150 are known Cadinadien found in the hop oil, Muuroladien in Terpentinsorten, Cadinen in Kubebenpfeffer and juniper . The Artemisiasäure is antibacterial. There are over 400 guajanes and cycloguajanes . Guajadiene is found in tolu balsam . A number of Pseudoguajanen, such as the Ambrosia acid is found in Ambrosia species . Many of the Himachalans are found in cedar oil . Several Daucans can be found in the wild carrot (Daucus carota), after which they are named. The by Marasman derived Isovelleral acting antibiotic from the Isolactaran derived Merulidial is a metabolite of Gallertfleischigen Fältlings ( Phlebia tremellosa ), a fungus which is found on dead wood. The acorans , the 50 chamigrans and the small group of axanans are spiro compounds , the chamigrans are found in algae. The from Cedran derived cedrol is fragrance of cedar oil. Hirsutans are often metabolites of fungi, one example is hirsutic acid . The tricyclic Spathulenol is found in mugwort ( Artemisia vulgaris ), tarragon ( Artemisia dracunculus ), real chamomile and other Artemisias , in cotton species ( Gossypium ), Hypericum perforatum and in various Nepeta species.
There are also some groups of polycyclic sesquiterpenes that are not derived from farnesan.
Diterpenes
The diterpenes are based on four isoprene units, i.e. a basic structure with 20 carbon atoms. There are around 5000 known diterpenes, all diterpenes are derived from the starting compound ( E , E , E ) - geranylgeranyl pyrophosphate .
Acyclic
Phytan is found, for example, together with phytanic acid in oil shale or in the human liver . Chlorophyll is an ester of phytol .
Cyclophytanes to Tetracyclophytanes
Most cyclophytanes are derived from 10,15-cyclophytane, 1,6-cyclophytanes are less common.
Retinal , retinol , axerophthene and tretinoin are representatives of the vitamin A series; retinal is bound to rhodopsin with the opsin in the retina of the eye and is important there for the visual process. The retinoic acids formed from retinal exert an influence on growth and cell differentiation. Agelasin E and Agelasidin B have an antispasmodic and antibacterial effect.
The approximately 400 bicyclophytanes are derived from labdane , more rarely from 1,6-cyclophytane, haliman or clerodane . Pumil oxide and abienol are found in spruce trees. Labdanolic acid and oxocativic acid are found in pine trees , sclareol in sage species, and pinifolic acid in the needles of pine trees.
Important parent compounds of the tricyclophytanes are the Primarane , Cassane, Cleistanthane and Abietane . The Primarane, such as podocarpinol , podocarpic acid and nimbiol , are mainly found in European pines. Of the Cassanen, mainly from the Cassainsäure , ester guided alkaloids from. Examples of cleistanthans are auricular acid and cleistanol . Abietans are mainly found in conifers, such as the resin acid abietic acid , abietenol or palustric acid .
The terpene alkaloid paclitaxel from the Pacific yew tree , derived from the tricyclic diterpenoid baccatin III , is also used as a cytostatic agent in cancer therapy due to its mitosis- inhibiting effect . Forskolin , which is also tricyclic , is used in biochemistry as an activator of adenylyl cyclase .
The tetracyclophytans are also divided into several stem groups. A kauran , 1,7,14-trihydroxy-16-kauren-15-one, has anti-tumor effects. The atisans , such as the atisen , can be converted into certain alkaloids . Of the Gibberellanen derived terpenoids such as gibberellic influence as plant hormones plant growth. The grayanotoxans , such as leucothol C, are often toxic and can be found in many leaves.
Sesterterpenes
The sesterterpenes are based on five isoprene units, i.e. a basic structure with 25 carbon atoms. Sesterterpenes were first isolated from insect wax and lower fungi in 1965. There are around 150 known sesterterpenes, 30 of which have a furan body; they are derived from 3,7,11,15,19-pentamethylicosane. Sesterterpenes are rather rare in nature; they are mainly found in lower plants, mushrooms or in the leaves of potatoes. Sesterterpenes with furan bodies can be isolated from sponges such as, for example, the species Ircinia campana .
The most important acyclic sesterterpenes are 3,7,11,15,19-pentamethyl-2,6-icosadien-1-ol, ircinin I and 8,9-dehydroircinin I. Ircin I has an antibacterial effect and is found, for example in the sponge Ircinia oros , the 8,9-dehydroircinin I in Cacospongia scalaris . The monocyclic sesterterpenes are found in sponges and waxes of insects of Ceroplastes ceriferus . Mention should be made of the cyclohexane sesterterpene , neomanoalide , which has an antibacterial effect, cericeran and cerifeol 1 . Bicyclic sesterpenes are, for example, dysideapalum acid , salvisyriacolid and salvisyriacolid methyl ester . The tricyclic sesterterpene cheilanthatriol is found in ferns. The tetracyclic sesterterpenes are found in sponges and are mainly based on the scalaran .
Triterpenes

The triterpenes are based on six isoprene units, i.e. a basic structure with 30 carbon atoms. There are about 1700 triterpenes; they are mainly derived from squalane , which consists of two tail-tail linked sesquiterpene units, as well as the squalene derived from it . D-vitamins and bile acids are oxidized derived triterpenes, the tetracyclic of the gonan is derived from steroids . Acyclic triterpenes are relatively rare in nature, especially tetracyclic and pentacyclic triterpenes are common in nature.
Tetracyclic
The tetracyclic triterpenes have the gonane skeleton as a basic chemical structure, which can also be found in the steroids , parent compounds are the Protostane and Fusidane , Dammarane , Apotirucallane , Tirucallane and Euphane , Lanostane , Cycloartane and Cucurbitane . Some terpenoids derived from the fusidans, such as fusidic acid , selectively intervene in the bacterial metabolism and are therefore used as antibiotics . An important Apotirucallan is the Melianin A . There are around 200 lanostanes, one example being the lanosterol . There are around 120 of the cycloartanes, while pineapple acid is found in pineapple wood . There are only about 40 natural cucurbitans, cucurbitacins F and B are being tested as chemotherapeutic agents in cancer therapy .
Pentacyclic
One of the basic structures of the pentacyclic triterpenes is the hopane (see hopanoids ). The Fernáne, Adianane and Filicane are mainly found in ferns, with some gammacerans also found in ferns. The Adianans and Filicans include, for example, the Simiarenol and Filicenal , which occurs in maidenhair fern , a Gammaceran is the Ketohakonanol . There are also several other pentacyclic triterpenes such as the stictans , serratans, and iridals . Stictane can be found in the bark of many trees, the Serratan 14 serrats are found in European forest ferns. A Iridal is the ambrein from Ambra of the sperm whale . However, most iridals are mainly found in irises . Betulin and betulinic acid (which inhibits HIV ) are found in the bark of birch trees .
Tetraterpenes
The tetraterpenes are based on eight isoprene units, i.e. a basic structure with 40 carbon atoms. The naturally occurring tetraterpenes include most of the carotenoids , which are widely used as fat-soluble pigments (lipochromes) in archaea , bacteria, plants and animals . These include the various carotenes , pure hydrocarbons such as lycopene with the empirical formula C 40 H 56 , and also their oxygen-containing derivatives, the xanthophylls . Some modified compounds with an elongated or shortened carbon structure, such as apocarotenoids or diapocarotenoids such as crocetin , are also referred to as carotenoids , but are not tetraterpenoids.
Polyterpenes
Polyterpenes consist of more than eight isoprene units; Leopold Ružička called these polyisoprenes well polyprenes .
cis polyisoprene is found in natural rubber , which is commercially obtained from the latex of the rubber tree and from that of the guayule plant. It is also contained in the latex of many other plant species, e.g. B. in the Russian dandelion and in the rubber tree .
trans -polyisoprene is the main component of the rubbery part of gutta-percha and balata as well as the "rubber" of the rubber elm and is less important commercially. Chicle , obtained from the sapling apple tree , contains a 1: 1 to 4: 1 mixture of trans and cis polyisoprene.
The polyterpenes also include long-chain polymers made up of isoprene subunits with a terminal hydroxyl group , called polyprenols . In contrast to bacterial polyprenols, the eukaryotic ones, like those of archaea, are α- unsaturated in the isoprenoid carrying the functional OH group . These include, for example, vegetable betulaprenols and dolichol in humans , which u. a. occurs as a lipid component in the neuromelanin of the substantia nigra .
Prenylquinones are terpenoids with up to ten isoprene units, including vitamins K 1 and K 2 , vitamin E , plastoquinone and ubiquinones .
literature
- Eberhard Breitmaier: Terpenes . Teubner Verlag, January 1999, ISBN 3-519-03548-0 .
- Lutz Roth: terpenes, turpentine oil . Ecomed Verlag, Landsberg, June 2001, ISBN 3-609-69140-9 .
- Gerhard Habermehl, Peter E. Hammann, Hans C. Krebs, natural product chemistry. 2nd edition, Springer Verlag, 2002, ISBN 3-540-43952-8 .
- Peter Nuhn: Natural Products Chemistry. Microbial, vegetable and animal natural substances. 2nd edition, S. Hirzel Wissenschaftliche Verlagsgesellschaft Stuttgart, 1990, ISBN 3-7776-0473-9 .
- JD Conolly, RA Hill: Dictionary of Terpenoids . Chapman & Hall, London, New York, Tokyo, Melbourne, Madras 1991.
Web links
Individual evidence
- ↑ a b c d e f E. Breitmaier: Terpenes - aromas, fragrances, pharmaceuticals, pheromones , 1st edition, BG Teubner, Stuttgart, Leipzig 1999, ISBN 3-519-03548-0 .
- ↑ Peter Nuhn : Natural Products Chemistry: Microbial, Plant and Animal Natural Products , 4th, revised. Edition, Hirzel S. Verlag, Stuttgart, ISBN 3-7776-1363-0 .
- ↑ Georg Sticker : Healing effects of terpenic oils and resins. Vienna and Leipzig 1917.
- ^ Hager's Handbook of Pharmaceutical Practice - 4th Edition .
- ↑ IUPAC definition of terpenes and terpenoids .
- ↑ A. Kekulé (1863) textbook of organic chemistry. Published by Ferdinand Enke, Erlangen .
- ^ O. Wallach: On the knowledge of the terpenes and the essential oils . In: Justus Liebig's Annals of Chemistry . tape 227 , no. 3 , 1885, p. 277-302 , doi : 10.1002 / jlac.18852270306 .
- ↑ a b c L. Růžička: Perspectives on biogenesis and the chemistry of terpenes . In: Pure and Applied Chemistry . tape 6 , no. 4 , 1963, pp. 493-524 , doi : 10.1351 / pac196306040493 .
- ↑ O. Wallach Nobel Prize Lecture (PDF, English; 82 kB) .
- ↑ L. Ruzicka Nobel Prize Lecture (PDF, English; 537 kB) .
- ^ Dissertation on structure elucidation by Cedren, GW Kusserow ETH Zurich 1948
- ↑ O. Wallach: Terpenes and Camphor: Summary of own investigations on d. Area d. alicyclic carbon compounds terpenes and camphor: summary of own investigations on d. Area d. alicyclic carbon compounds, Veit Leipzig 1914. DNB 361835116 .
- ^ A. de Meijere: Adolf von Baeyer - Nobel Prize Winner for Chemistry 1905, Angew. Chem. , 2008 , 117 , pp. 8046-8050; doi: 10.1002 / anie.200503351 .
- ^ FW Semmler: On the knowledge of the components of essential oils. (Constitution of the α-Santalol and α-Santalen series: The constitution of the sesquiterpene alcohols and sesquiterpenes.). In: Reports of the German Chemical Society. 43, 1910, p. 1893, doi: 10.1002 / cber.191004302121 .
- ↑ a b Nobel Prize lecture by F. Lynen (PDF; 536 kB).
- ↑ Nobel Prize lecture by K. Bloch (PDF; 191 kB).
- ↑ M. Rohmer: The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants , in: Nat Prod Rep . , 1999 , 16 , pp. 565-574; doi: 10.1039 / A709175C .
- ↑ H. Kleinig: The Role of Plastids in Isoprenoid Biosynthesis . In: Annual Review of Plant Physiology and Plant Molecular Biology . tape 40 , June 1989, pp. 39-59 , doi : 10.1146 / annurev.pp.40.060189.000351 ( PDF ).
- ↑ Patent EP0776202 : Liposomal encapsulated taxol, its production and its use. Registered on August 18, 1995 , published on May 17, 2000 , applicant: Max Delbrück Center for Molecular Medicine, inventors: Martin Brandl, Iduna Fichtner, Regine Reszka, Gernot Warnke.
- ↑ Patent EP1471886 : Process for increasing the water solubility of lipophilic active ingredients; Production of highly concentrated aqueous compositions of these active ingredients; such products and their uses. Registered on January 15, 2003 , published on August 15, 2007 , applicant: KliniPharm GmbH, inventors: Eva Bogdanovic, Katja Grzimek, Wolfgang Schatton.
- ↑ Federal Environment Agency: There's something in the air - about fragrances in the public and private sector , accessed on January 3, 2020.
- ↑ M. Dobler, J: D. Dunitz, B. Gubler, HP Weber, G. Büchi, J. Padilla O .: The Structure of Patchouli Alcohol . In: Proceedings of the Chemical Society . No. 12 , 1963, pp. 383 , doi : 10.1039 / PS9630000357 .
- ^ E. von Rudloff: Gas-Liquid Chromatography of Terpens . In: Canadian Journal of Chemistry . 30, 1961, pp. 1200-1206, doi : 10.1139 / v61-152 .
- ↑ Description of a school experiment .
- ↑ E. Stahl, W. Schild: Isolation and characterization of natural substances . Gustav Fischer Verlag, Stuttgart 1986, ISBN 3-437-30511-5 .
-
↑ Patent for the large-scale synthesis of menthol:
Patent EP1162186 : Process for the production of d, l-menthol. Registered on April 30, 2001 , published on December 12, 2001 , applicant: Bayer AG, inventors: Claus Dreisbach, Michael Friederich, Hans-Jürgen Gross, Jörg-Dietrich Jentsch, Gerald John, Reinhard Langer, Thomas Prinz, Andreas Schlemenat, Andreas Schulze-Tilling. -
↑ Patent for the large-scale synthesis of camphor:
Patent EP0539990 : Process for the production of camphene by rearrangement of alpha-pinene. Registered on October 29, 1992 , published on January 8, 1997 , applicant: Hoechst Aktiengesellschaft, inventors: Eberhard Ritter, Thomas Wisser, Alfred Riedel, Manfred Gscheidmeier, Joachim Maginot. -
↑ Example for the isolation of hemiterpenes as glycosides:
Søren Damtoft, Søren Rosendal Jensen: Hemialboside, a hemiterpene glucoside from Lamium album . In: Phytochemistry . tape 39 , no. 4 , July 1995, p. 923-924 , doi : 10.1016 / 0031-9422 (95) 00085-L . - ↑ Bernhard Watzl: Monoterpenes . In: Nutrition review . tape 49 , no. 8 , 2002, p. 322-324 ( PDF ).
- ^ T. Graham, E. Gray, J. Burgess, B. Goess: An Efficient Synthesis of (±) -Grandisol Featuring 1,5-Enyne Metathesis. In: Journal of Organic Chemistry. Volume 75, No. 1, 2010, pp. 226-228; doi : 10.1021 / jo9020375 , PMC 2798917 (free full text).
- ↑ K. Langer, J. Mattay, A. Heidbreder, M. Möller: A New Stereoselective Synthesis of Grandisol. In: European Journal of Organic Chemistry. March 1992, pp. 257-260, doi: 10.1002 / jlac.199219920144 .
- ↑ www.kliniken.de: Iridoids. ( Memento from August 2, 2012 in the web archive archive.today )
- ↑ Entry on turpentine oil. In: Römpp Online . Georg Thieme Verlag, accessed on May 25, 2014.
- ↑ L. Tschugaeff: Ueber das Thujen, a new bicyclic terpene , in: Reports of the German Chemical Society , 1900 , 33 , pp. 3118-3126; doi: 10.1002 / cber.19000330363 .
- ↑ Ziaei, A. et al .: Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects , in: Phytother Res , 2011 , 25 (4), pp. 557-562; doi: 10.1002 / ptr.3289 ; PMID 20857430 .
- ^ GW Elzen, HJ Williams, SB Vinson: Isolation and identification of cotton synomones mediating searching behavior by parasitoid Campoletis sonorensis . In: Journal of Chemical Ecology . tape 10 , no. 8 , August 1984, p. 1251-1264 , doi : 10.1007 / BF00988552 .
- ↑ KHC Baser, T. Ozek, HR Nuriddinov, AB Demirci: Essential Oils of Two Hypericum Species from Uzbekistan . In: Chemistry of Natural Compounds . tape 38 , no. 1 , January 2002, p. 54-57 , doi : 10.1023 / A: 1015781715535 .
- ↑ KHC Baser, N. Kirimer, M. Kurkcuoglu, B. Demirci: Essential Oils of Nepeta Species Growing in Turkey . In: Chemistry of Natural Compounds . tape 36 , no. 4 , July 2000, p. 356-359 , doi : 10.1023 / A: 1002832628159 .
- ↑ Eberhard Breitmaier: Terpenes: flavors, fragrances, pharmaceuticals, pheromones. 2nd edition, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 52, doi: 10.1002 / 9783527623693 .
- ↑ Shinzo Kohjiya, Yuko Ikeda: Chemistry, Manufacture and Applications of Natural Rubber. Woodhead, 2014, ISBN 978-0-85709-683-8 , pp. 30–34, limited preview in Google Book Search.
- ↑ Charles E. Carraher Jr., LH Sperling: Polymer Applications of Renewable resource material. Plenum Press, 1983, ISBN 978-1-4613-3505-4 (Reprint) p. 9.
- ↑ A. Behr, Th. Seidensticker: Introduction to the chemistry of renewable raw materials. Springer, 2018, ISBN 978-3-662-55254-4 , p. 234, limited preview in the Google book search.
- ↑ A. Steinbüchel, T. Koyama: Biopolymers. Volume 2: Polyisoprenoids , Wiley, 2001, ISBN 978-3-527-30221-5 , p. 11.
- ↑ H. Fedorow, R. Pickford, J. Hook, K. Double, G. Halliday, M. Gerlach, P. Riederer, B. Garner: Dolichol is the major lipid component of human substantia nigra neuromelanin . In: Journal of Neurochemistry . 92, No. 4, February 2005, pp. 990-995. doi : 10.1111 / j.1471-4159.2004.02975.x .