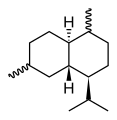
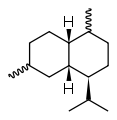
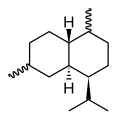
Cadinene

The cadinenes (stress on the third syllable: cadin e ne ) are a group of chiral compounds that differ in the position of the double bonds in the molecule, the configuration of the stereocenters and the link between the two cyclohexane rings. They are unsaturated bicyclic hydrocarbons and belong to the series of sesquiterpenes . All of them are based on the structure of the saturated parent compound, cadinane .
The name is derived from Juniperus oxycedrus (French: Cade or Genévrier cade , stinging juniper ), from whose wood cadinene was isolated for the first time.
Depending on the stereochemical configuration, the individual representatives of the group of substances are also called Muurolene , Amorphene or Bulgarene referred.
Representative
The eight possible stereo isomers with three stereogenic centers are all known, the most important and at the same time the most common sesquiterpene is (-) - β-cadinene ( specific rotation : [α] D 20 = −251 °). It is a hexahydro derivative of 4-isopropyl-1,6-dimethylnaphthalene ( cadalin ), the saturated parent compound is known as cadinane.
| Cadinene | ||||||
| Surname | α-cadines | β-cadines | γ-cadines | δ-cadines | ε-cadines | |
| other names | (1 S , 4a R , 8a S ) -1-isopropyl-4,7- dimethyl-1,2,4a, 5,8,8a-hexahydronaphthalene |
γ-muuroles | δ-amorphs | ε-Muurolen | ||
| Structural formula |

|

|

|

|

|
|
| CAS number | 24406-05-1 | 523-47-7 | 1460-97-5 39029-41-9 |
483-76-1 19912-65-3 |
1080-67-7 2535-42-4 20307-98-6 29887-40-9 |
|
| ? (Mixture of isomers) | ||||||
| PubChem |
12306048 101708 |
3032853 |
15094 6429304 6432404 92313 10512446 6432308 5315591 |
10223 441005 12306055 |
5315590 520461 |
|
| Molecular formula | C 15 H 24 | |||||
| Molar mass | 204.39 g mol −1 | |||||
| Physical state | liquid | |||||
| Brief description | colorless oil with a pleasant odor | |||||
| Melting point | ||||||
| boiling point | 275 ° C | |||||
| density | 0.92 g cm −3 | |||||
| solubility | almost insoluble in water | |||||
|
GHS labeling |
|
|||||
| H and P phrases | see above | |||||
| see above | ||||||
| see above | ||||||
Stereochemistry
Depending on the linkage of the two cyclohexane rings of decalin , the individual representatives of the substance group are also referred to as muurolene, amorphous or bulgarene , depending on the absolute stereochemical configuration.
Cadinenes are sensitive to light and should therefore be stored in dark places.
Occurrence and extraction

(-) - β-Cadinen is found in the essential oil of the cubeb pepper and in the leaves of some germander species. It is the main component of Cade oil , which is obtained by dry distillation of juniper wood ( Juniperus oxycedrus ) in the Mediterranean region. Its isomer γ-cadinen occurs in goldenrod species. Cadinene could also be detected in the propolis produced by bees .
use
- Ointments for skin rashes
- Tar soaps
- Hair ointments
- β-Cadinen is used as a flavoring in baked goods, candy and chewing gum and as a fragrance in cosmetics and detergents.
Individual evidence
- ↑ a b c d e Entry on Cadinen. In: Römpp Online . Georg Thieme Verlag, accessed on June 8, 2014.
- ↑ With regard to its dangerousness, the substance has not yet been classified by the EU, a reliable and citable source has not yet been found.
- ↑ F. Pellati, FP Prencipe, S. Benvenuti: Headspace solid-phase microextraction-gas chromatography-mass spectrometry characterization of propolis volatile compounds. In: Journal of Pharmaceutical and Biomedical Analysis . Volume 84, October 2013, pp. 103–111, doi : 10.1016 / j.jpba.2013.05.045 . PMID 23807002 .