Thioterpineol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Simplified structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thioterpineol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 18 S | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 170.31 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.948 g cm −3 (20 ° C) [( R ) form)] |
||||||||||||||||||
| boiling point |
40 ° C (0.13 hPa ) |
||||||||||||||||||
| Refractive index |
1.503 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
The monoterpene thioterpineol ( p- menth-1-en-8-thiol) is a flavor component of grapefruit juice and the substance with the lowest known odor threshold .
Already about 10 −4 ppb of the racemate can be perceived in aqueous solution (see below for the differences in odor intensity).
Thioterpineol is one of the sulfur analogues of alcohols, the thiols .
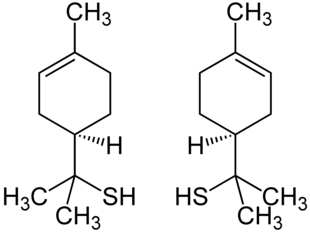
Thioterpineol contains a stereocenter, so it is chiral . Thus, there are two mirror-image stereoisomers of thioterpineol: ( R ) -thioterpineol and ( S ) -thioterpineol. The racemate [1: 1 mixture of ( R ) -thioterpineol and ( S ) -thioterpineol] is called ( RS ) -thioterpineol.
( R ) -Thioterpineol (left) and ( S ) -Thioterpineol (right)
The enantiomers differ in terms of their odor threshold . For the ( S ) enantiomer it is 0.00008 ppb, for the ( R ) form it is only 0.00002 ppb. The odor qualities also differ: the ( S ) -enantiomer smells more fruity and less sulphurous than the ( R ) -enantiomer.
Individual evidence
- ↑ Entry on TRIMETHYL-3-CYCLOHEXENE-1-METHANETHIOL in the CosIng database of the EU Commission, accessed on April 16, 2020.
- ↑ a b c d Entry on p-menth-1-en-8-thiol. In: Römpp Online . Georg Thieme Verlag, accessed on March 26, 2016.
- ↑ There is not yet a harmonized classification for this substance . A labeling of α, α, 4-trimethylcyclohex-3-ene-1-methanethiol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 29, 2019, is reproduced from a self-classification by the distributor .
- ↑ Beyer-Walter: Textbook of organic chemistry
- ^ John C. Leffingwell: Chirality & Odor Perception - The p-Menthen-8-thiols .