Vitamin A
Vitamin A denotes several chemical compounds that have biological functions in all animals . Some of them are ingested directly with food or formed from carotenes (provitamin A) , which not all animals are capable of ( domestic cats, for example).
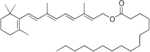
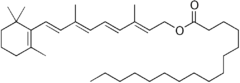
In humans, retinal (synonym: vitamin A aldehyde), retinol (vitamin A 1 ), retinoic acids (vitamin A acids) and retinyl palmitate (vitamin A ester) are counted as vitamin A and 3-dehydroretinol including aldehyde. They can be converted into one another by enzymatically catalyzed reactions, with the only exception that retinoic acids can no longer be recycled. Chemically, they are retinoids . If there is not enough of it in the body, hypovitaminosis develops .
history
Already around 1500 BC The Chinese used liver and honey to cure night blindness . Guilleaume described this healing in the 16th century AD.
At the beginning of the 20th century, the influence of different diets on the growth of mammals such as rats and mice was investigated. In 1912 Gowland Hopkins discovered that a lack of essential compounds, which he called “accessory food factors”, led to significant growth disorders: “It is possible that what is absent from artificial diets and supplied by such addenda as milk and tissue extracts is of the nature of an organic complex (or of complexes) which the animal body cannot synthesize. ”He suspected a connection between the undersupply of these substances and similar health problems in humans, but did not investigate this further.
In 1913 Elmer McCollum - and independently Mendel with Osborne - succeeded in isolating the fat-soluble retinol . In 1916, McCollum introduced the categorization of vitamins by letter, in which he initially referred to retinol as "Fat-Soluble Factor A". In 1920 the name became "Vitamin A", using the term coined by Casimir Funk ("Vital-Amin").
Occurrence
In animal foods, vitamin A is mainly available as retinyl palmitate, in vegetable foods as carotenes. The contents are therefore given uniformly as retinol equivalent (RAE).
| food | origin | Retinol equivalent µg / 100 g |
|---|---|---|
| Cod liver oil | animal | 30000 |
| Beef liver | animal | 7744 |
| Liver sausage | animal | 4220 |
| Chicken liver | animal | 3980 |
| sweet potato | vegetable | 0-1000 |
| Carrot juice | vegetable | 950 |
| Carrots , raw | vegetable | 800-850 |
| Carrots , cooked | vegetable | 500-800 |
| Canned Pumpkin | vegetable | 780 |
| Kale , cooked / frozen | vegetable | 730 |
| butter | animal | 680 |
| Breakfast cereal | vegetable | 300-500 |
| Spinach , raw | vegetable | 470 |
| Pith cabbage leaves , cooked | vegetable | 400 |
| egg yolk | animal | 380 |
| Dandelion leaves , cooked | vegetable | 300 |
| Pumpkin , cooked | vegetable | 250 |
| Apricots , raw / dried | vegetable | 100-200 |
| Corn , raw | vegetable | 185 |
| Cantaloupe melon , raw | vegetable | 170 |
| Oh , whole | animal | 140 |
| Apricot , canned | vegetable | 60-80 |
| Condensed milk | animal | 74 |
| Salmon , depending on the preparation | animal | 17-64 |
| milk | animal | 46 |
| Mango , raw | vegetable | 40 |
| Chicken | animal | 5-25 |
| Peach , raw | vegetable | 15-20 |
| Buttermilk , low in fat | animal | 14th |
| Cod , raw | animal | 14th |
| Cod, fried | animal | 10 |
| Pork acc. To Zub. | animal | 0-10 |
The body can hardly break down excess vitamin A, which is why it easily accumulates in the body, especially in the liver. Therefore, the liver of animals contains so much vitamin A that frequent consumption of the liver in turn leads to an accumulation in the body of the consumer. This can lead to hypervitaminosis . Pig liver, for example, contains up to 42 mg of vitamin A (140,000 IU) per 100 g. The liver of the polar bear ( Ursus maritimus ) is toxic due to its very high vitamin A content and is therefore not eaten by the Eskimos . This is also true, to a lesser extent, of other Arctic animals , particularly various seals .
Synthetic manufacture
Vitamin A is also produced synthetically to a considerable extent as retinaol acetate, the process is based on citral , which is converted to β-ionone , from which a building block with 15 carbon atoms is produced by adding ethyne and converted to a phosphorus ylide (Wittig salt) is implemented. This reacts in a Wittig reaction with β-formylcrotyl acetate to form retinol acetate.
physiology
Provitamin A (carotenes), retinyl palmitate and retinol are ingested with food. Retinyl palmitate is hydrolyzed to retinol by pancreatic lipase . Although the carotenes can be converted into vitamin A in most tissue types, most of this conversion takes place in intestinal cells. The resulting retinal and retinol are absorbed in the cytosol by cytosolic retinol-binding proteins (CRBP I to III), in turn converted into retinyl palmitate and transported to the liver with the help of chylomicrons . From there they are transported in the plasma with the help of the plasma RBP. The RBP receptor facilitates reception at the target cell . Retinol / retinal can be temporarily stored in the tissue as retinyl palmitate ; this is also the storage form in which most of the vitamin A is in the liver.
Digestion and absorption of vitamin A.
Retinyl palmitate and retinol are ingested with animal food. Retinal and retinoic acid, on the other hand, play no role in nutrition. In any case, they are lipophilic compounds that collect in the intestine along with other lipids . Retinol binds directly to the cell membrane of enterocytes ; retinyl palmitate is previously broken down into retinol and palmitate using the enzyme pancreatic lipase :
Since retinol binds to the retinol-binding protein CRBP II located inside the cell more strongly than to the membrane, retinol does not stay in the membrane for long and moves into the cytosol.
Conversion of the carotenes
In addition to β-carotene , animals and humans ingest vegetable α-carotene and β-cryptoxanthin with their food. The enzyme β-carotene-15,15'-monooxygenase (BMO) is able to convert these carotenes into retinal, whereby only with β-carotene this conversion to two molecules of retinal takes place completely, while the other substances are split asymmetrically and each only one molecule of retinal is produced.
β-carotene is split into two molecules all-trans -retinally. BMO is expressed in many tissue types, but most activity is in the intestine due to the availability of substrate.
The retinal from this reaction also binds quickly to CRBP II inside the enterocytes. If the vitamin A needed breastfed, which is BCMO1 - gene expression scaled back. Excess β-carotene is localized in lipophilic zones of the body, including the skin, which in extreme cases can be perceived as a harmless yellow color ( aurantiasis cutis , carotenemia).
Retinal is then reduced to retinol, probably by an enzyme that is localized in the membrane of the ER (facing the cytosol), the retinal reductase RalR1.
How much of the ingested β-carotene a person can actually convert into vitamin A depends on their genetic situation. Large parts of the population carry variations on at least one of the two BCMO1 genes that make the conversion - which is already ineffective (only 3% or less of the carotenoids ingested are absorbed, the conversion is then only a factor of 28: 1 beta-carotene in some plants) to retinol) - by up to 70%. For some people it is almost impossible to meet the vitamin A requirement through a purely plant-based diet.
Esterification and transport to the liver
Retinol that is not required is esterified to retinyl palmitate in many tissue types; the catalyzing enzyme is lecithin retinol acyltransferase :
All-trans -retinol and dipalmitoyl lecithin are converted to retinyl palmitate and 2-palmitoyl lecithin. The lecithins are taken from the ER membrane, in the vicinity of which the reaction takes place.
The microsomal triglyceride transfer protein is now required for incorporation into chylomicrons . It is not known how many molecules of retinyl palmitate ultimately make their way in a chylomicron, which first passes through the lymph and then into the blood plasma. Since there are no known transfer proteins for retinyl palmitate, it remains in chylomicrons up to the liver. Their degradation there in the endothelium of liver parenchymal cells by lipoprotein lipase and the immediate hydrolysis there by a retinyl ester hydrolase (REH) leads to the uptake of retinol in the cytosol of liver cells.
function
Vitamin A is important for the growth, function and structure of the skin and mucous membranes , blood cells , metabolism and the visual process . The utilization of this vitamin in the body can be disrupted by liver damage and the intake of estrogen preparations. The latest studies have shown that, contrary to the assumption, even the smallest amounts of fat in food can absorb and use vitamin A by the body.
Nervous system
Retinol maintains healthy nerve cells in the peripheral nerve pathways , in the brain and in the spinal cord .
Blood cells
Retinol decisively promotes the formation of new erythrocytes and facilitates the incorporation of iron .
Protein metabolism
It is involved in protein synthesis and lipid metabolism in the liver, so a protein-rich diet can lead to vitamin A deficiency. Even with increased stress, the vitamin A requirement increases, since stress increases the protein requirement. This means that the need for retinol also increases in serious illnesses such as arthritis , AIDS or cancer .
Skin and mucous membranes
Vitamin A plays a central role in the structure and health of these tissues, as it ensures normal cell growth not only in the skin but also in the walls of the respiratory , digestive and urinary tracts . It also prevents DNA damage in skin cells, contributes to their repair and normalizes skin functions, for example healthy cell division of keratinocytes (see epidermis ).
Skeleton
Vitamin A is involved in ossification , bone formation and bone healing . A sufficient supply of vitamin A is therefore particularly important for children.
Embryonic growth
The vitamin A acid (all-trans retinoic acid) or its salt Retinat , is an important growth factor for nerve cells during embryonic development. It is secreted by cells of the primitive node and is involved in the formation of the longitudinal axis (front-back orientation) of the embryo. Nerve cells migrate along the retinoic acid concentration gradient.
reproduction
Retinol is involved in the synthesis of testosterone and estrogen as well as in spermatogenesis and oogenesis, as well as in the synthesis of retinal, a component of rhodopsin, the protein responsible for the perception of light in the photoreceptors of the eyes. Furthermore, the amount and shape of sperm depends on an optimal supply of vitamin A. In addition to its effects on the human mucous membranes, vitamin A is also important for maintaining the structure and function of the spermatic and fallopian tubes (both lined with mucous membrane). In women, infertility and miscarriages are associated with retinol deficiency.
immune system
On the one hand, retinol increases the resistance to infections because, as already mentioned, vitamin A keeps the skin and mucous membranes healthy and thus supports effective barriers against bacteria, viruses and parasites. Furthermore, retinol and beta-carotene increase the effectiveness and number of white blood cells and also facilitate the production of antibodies . Even a slight deficiency increases the risk of developing pneumonia or diarrhea by two to three times.
requirement
The actual daily requirement depends on age, gender and living conditions. Adults should take in an average of 0.8 to 1.0 mg (= 2,600–3,300 IU) daily, with men having a slightly higher requirement than women. Long-term cooking, oxygen and light damage vitamin A. Therefore, foods containing vitamin A should always be stored unpeeled or wrapped and in the dark - preferably in the refrigerator. Cooking losses are between 10 and 30 percent.
defect
A lack of vitamin A ( vitamin A deficiency ) leads to increased susceptibility to infection, dryness of the skin, hair, nails and eyes ("eye strain", xerophthalmia ), hair loss, night blindness , reduced visual acuity, increased sensitivity to light, keratomalacia , iron deficiency, increased Risk of arteriosclerotic heart disease, increased risk of cancer in organs with mucous membranes, increased risk of kidney stones due to increased calcium excretion, fertility disorders, impaired sense of smell, sense of touch and appetite, fatigue and growth disorders such as e.g. B. Bone growth disorders in childhood.
Causes of Hypovitaminosis
- Inflammation, surgery, but also stress
- Smoking and constant breathing of bad air
- Environmental toxins such as B. Cadmium
- Strong sunlight (e.g. on the beach or in the snow), especially for fair-skinned people
- Disorders of fat absorption, mostly due to problems with the liver, gallbladder or pancreas (e.g. in the case of exocrine pancreatic insufficiency )
- Around one in five Europeans does not get enough retinol through their diet. An undersupply can quickly develop, especially with children, since children have fewer storage options, but have a high need.
- Alcohol affects absorption, storage and mobilization
- Diabetics and people with hyperthyroidism have difficulty converting vegetable carotenoids into vitamin A.
- Cholesterol lowering agents and laxatives worsen absorption
- Certain sleeping pills use up stores in the liver
The shortage of vitamin A ( vitamin A deficiency , VAD for short, also A-avitaminosis ) is a widespread problem in developing countries. Approximately 250 million preschool children suffer from VAD and approximately one million children die from it each year. Between 250,000 and 500,000 children also go blind from VAD and half die in the following year. Vitamin A deficiency also leads to a greatly increased complication rate in infectious diseases such as measles . For children with measles, the WHO recommends two doses of vitamin A, which can prevent measles-related blindness or eye damage, and also reduce mortality . In contrast, vitamin A doses are not suitable for the prevention of measles.
Countermeasures
There are several strategies to avoid vitamin A deficiency, which occurs primarily as a phenomenon of poverty in developing countries:
- Distribution of vitamin tablets: Retinol tablets are typically administered every 6 months. The retinol is stored in the liver and released from there over a period of four to six months. This strategy is cost effective, but it can be difficult to reach large proportions of children in need.
- Food fortification : Here, foods are fortified with micronutrients during manufacture or packaging. In Latin America, for example, sugar fortification has made a significant contribution to combating VAD. However, fortification is only an option when those in need are consuming processed products. In Africa this is e.g. B. is often not the case.
- Diversification of diet: Measures such as education among those affected should help to ensure that more vitamin A-rich foods are consumed, for example from their own garden. The disadvantage is that the availability of foods rich in vitamin A often fluctuates strongly depending on the season.
- Biofortification : With the help of plant breeding, the micronutrient content of crops is increased. The content of provitamin A, zinc and iron in staple foods such as cassava, corn, rice and sweet potatoes has been increased, also with the help of genetic engineering ( golden rice ). It is estimated that biofortification is cost-effective in developing countries.
Oversupply
In contrast, a (prolonged) oversupply of vitamin A can lead to vomiting, diarrhea, headaches, increased intracranial pressure ( pseudotumor cerebri ), decrease in bone tissue density ( osteoporosis ), enlargement of the liver and spleen, reduction in thyroid activity and painful growths of the periosteum. In healthy, non-pregnant women, higher single doses can be regarded as harmless, while repeated higher doses carry the risk of intoxication. For daily doses from 7.5 mg (= 25,000 IU) upwards and intake of several years, cases of liver cirrhosis have been described, some of them resulting in death. In the event of a massive overdose, the cone epiphyses are usually symmetrical on the thigh bone .
An oversupply of vitamins during pregnancy can already be assumed with a daily intake of vitamin A 10,000 IU or 3 mg per day, which can lead to childhood malformations such as craniofacial abnormalities or heart valve defects and spontaneous miscarriages .
A single study that showed teratogenic properties in the intake of 30,000 IU of vitamin A could not be confirmed. The safety of a dose of 10,000 IU has been shown several times. The recommendation of a daily dose of 2,500 IU (0.75 mg) seems to be justified.
The earliest evidence of hypervitaminosis A was discovered in Africa on the 1.7 million year old female skeleton KNM-ER 1808 of a Homo erectus , which showed the types of bone malformations typical of hypervitaminosis A, presumably caused by the consumption of extremely large amounts of Liver.
The oversupply of carotenes does not lead to an oversupply of vitamin A (hypervitaminosis A) in humans, because the body downregulates the conversion of carotenes to vitamin A. Too much carotenes is visually noticeable as yellowing of the skin ( carotenemia , "carrot terus "), but does not require treatment because there is no hypervitaminosis.
Diagnosis
The serum level is unsuitable for diagnosing hypervitaminosis. A reliable indicator, however, is the ratio of vitamin A to RBP ( retinol-binding protein ). If the serum level exceeds the binding capacity of RBPs, free vitamin A is present, which has a toxic effect.
Individual evidence
- ↑ Florian J. Schweigert et al .: Cats absorb beta-carotene, but it is not converted to vitamin A . In: The Journal of Nutrition . tape 132 , 6 Suppl 2, June 2002, p. 1610S – 2S , doi : 10.1093 / jn / 132.6.1610s , PMID 12042471 .
- ↑ Vitamin A, RAE (μg) Content of Selected Foods per Common Measure, sorted alphabetically . In: US Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory (Ed.): USDA National Nutrient Database for Standard Reference, Release 22 . 2009 ( usda.gov [accessed September 28, 2010]).
- ^ Dietrich Mebs: Gifttiere - A manual for biologists, toxicologists, doctors and pharmacists. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1992; Page 128, ISBN 3-8047-1219-3 .
- ↑ BASF expands vitamin A plant in Ludwigshafen Chemie.de from September 18, 2018, accessed on February 5, 2020
- ↑ Werner Reif, Hans Grassner: The technical vitamin A synthesis of BASF . In: Chemical Engineer Technology . tape 45 , no. 10 , 1973, p. 646-652b , doi : 10.1002 / cite.330450920 .
- ↑ a b R. Blomhoff et al .: Vitamin A metabolism: new perspectives on absorption, transport, and storage . In: Physiological Reviews . tape 71 , no. 4 , October 1991, p. 951-990 , doi : 10.1152 / physrev.1991.71.4.951 , PMID 1924551 .
- ↑ Earl H. Harrison and M. Mahmood Hussain: Mechanisms Involved in the intestinal digestion and absorption of dietary vitamin A . In: The Journal of Nutrition . tape 131 , no. 5 , May 2001, pp. 1405-1408 , doi : 10.1093 / jn / 131.5.1405 , PMID 11340090 .
- ↑ a b Yvette Fierce et al .: In vitro and in vivo characterization of retinoid synthesis from beta-carotene . In: Archives of Biochemistry and Biophysics . tape 472 , no. 2 , April 15, 2008, p. 126–138 , doi : 10.1016 / j.abb.2008.02.010 , PMID 18295589 , PMC 2587144 (free full text).
- ↑ Peter Altmeyer, M. Bacharach-Buhles, N. Buhles, Neal H. Brockmeyer, M. Herde, M. Stucker: Springer Encyclopedia Dermatology, Allergology, Environmental Medicine . Springer, ISBN 3-540-41361-8 , pp. 165-166 .
- ^ Sarah Ballantyne: Genes to Know About: Vitamin A Conversion Genes
- ↑ A Ruiz, A Winston, YH Lim, BA Gilbert, RR Rando, D Bok: Molecular and biochemical characterization of lecithin retinol acyltransferase . In: J. Biol. Chem. . 274, No. 6, February 1999, pp. 3834-3841. PMID 9920938 .
- ↑ Neeru Nayak et al .: Retinyl ester secretion by intestinal cells: a specific and regulated process dependent on assembly and secretion of chylomicrons . In: Journal of Lipid Research . tape 42 , no. 2 , February 2001, p. 272-280 , PMID 11181758 .
- ↑ Vitamin A, β-carotene. German Nutrition Society, accessed on May 16, 2020 .
- ↑ Micronutrient deficiencies, WHO, 2011.
- ↑ Lamine Traoré et al .: [Strategies to control vitamin A deficiency] . In: Sante (Montrouge, France) . tape 8 , no. 2 , March 1998, p. 158-162 , PMID 9642744 .
- ↑ Measles. World Health Organization , December 5, 2019, accessed February 5, 2020 .
- ↑ Rene F. Najera: Vitamin A and Measles. In: History of Vaccines. March 12, 2019, accessed February 5, 2020 .
- ↑ Hugo De Groote et al .: Estimating consumer willingness to pay for food quality with experimental auctions: the case of yellow versus fortified maize meal in Kenya . In: Agricultural Economics . tape 42 , no. 1 , 2011, p. 1–16 , doi : 10.1111 / j.1574-0862.2010.00466.x .
- ↑ André. P. Geubel et al .: Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases . In: Gastroenterology . tape 100 , no. 6 , June 1991, pp. 1701-1709 , doi : 10.1016 / 0016-5085 (91) 90672-8 , PMID 2019375 .
- ↑ Sabina Bastos Maia et al .: Vitamin A and Pregnancy: A Narrative Review . In: Nutrients . tape 11 , no. 3 , March 22, 2019, doi : 10.3390 / nu11030681 , PMID 30909386 , PMC 6470929 (free full text).
- ↑ Stefan Hartmann et al .: Exposure to retinyl esters, retinol, and retinoic acids in non-pregnant women Following Increasing single and repeated oral doses of vitamin A . In: Annals of Nutrition & Metabolism . tape 49 , no. 3 , May 2005, p. 155-164 , doi : 10.1159 / 000086879 , PMID 16006784 .
- ^ Richard K. Miller et al .: Periconceptional vitamin A use: how much is teratogenic? In: Reproductive Toxicology (Elmsford, NY) . tape 12 , no. 1 , January 1998, ISSN 0890-6238 , p. 75-88 , doi : 10.1016 / s0890-6238 (97) 00102-0 , PMID 9431575 .
- ↑ A. Walker et al .: A possible case of hypervitaminosis A in Homo erectus . In: Nature . tape 296 , no. 5854 , March 18, 1982, p. 248-250 , doi : 10.1038 / 296248a0 , PMID 7038513 .