Golden rice
Golden rice ( Golden Rice ) is a type of rice that contains increased amounts of beta-carotene ( provitamin A ) through genetic engineering . The development was initiated in 1992 by biologists Ingo Potrykus and Peter Beyer . The first results were published in Science in 2000 and, among other things, the subject of a cover story in Time magazine entitled This rice could save the lives of millions of children every year . The related concerns have not been met for various reasons.
According to Potrykus and Beyer, the aim of the project was to combat vitamin A deficiency, which is common in many developing and emerging countries . The golden rice is - depending on your point of view - a showcase project or a Trojan horse of transgenic green genetic engineering . Environmental and anti-globalization organizations criticized the golden rice. This led to increased reviews in individual countries, which can be found, among other things, in the Cartagena Protocol adopted in 2003 .
Regardless of this, the Golden Rice and the consortium it represents has received the US Patent Office's 2015 Patents for Humanity Award , which honors the application and release of patented technologies for global humanitarian applications. Beyer and Potrykus became known and honored worldwide for their commitment to the golden rice. Ongoing field research on golden rice is carried out in the USA, Vietnam and the Philippines, among others, the private Bill and Melinda Gates Foundation is the largest donor. A continuation is the ProVitaMinRice project , which endeavors to enrich the golden rice with other vital micronutrients .
backgrounds
In Southeast Asian regions, rice is the main food alongside milk. The white polished rice is valued more than the more nutritious unpeeled (hand milled) rice. White rice is preferred for aesthetic reasons and has higher prestige. The popularity of polished rice has negative health effects due to the lack of nutrients found in unpolished rice. In the case of low-fat malnutrition, the absorption of nutrients is restricted; rice itself is the main source of fat in the affected people. The substitution of conventional rice with provitamin-A-containing golden rice should reduce the vitamin A deficit. Critics argue that the golden rice may not be accepted because of its yellowish color. The complexity of the problem of malnutrition, which can be traced back to social, economic and cultural factors, suggests that the solution should not be sought in a single agricultural development. Golden rice would significantly reduce the vitamin A deficiency, but leave the other symptoms of malnutrition untouched and also leave the farmers dependent on the large agricultural corporations. According to Greenpeace , malnutrition and malnutrition are usually a sign of poverty. The consequences of the vitamin A deficiency would be better to remedy with a combination of the dispensing of high-dose vitamin A tablets, admixtures in staple foods and the promotion of fruit and vegetable gardens. Greenpeace relied on the recommendations of the World Health Organization (WHO) on conventional procedures. According to a study published in 2012, this complicates a monocultural orientation of the subsistence economy, a lack of infrastructure, accessibility and lack of education. According to the authors of the study, those affected can be reached more safely with modified seeds and more nutrient-rich varieties. Studies on the use of vitamin pills in Ethiopia have shown that these are helpful as a short-term crisis intervention, but that measures for a sustainable food supply must be followed, and that people with low incomes need to be supported with regard to nutrition. Even the (conventional) systematic fortification of staple foods still fails (2015) in some African countries due to the simplest conditions.
Field trials with golden rice were first carried out in 2004 in the USA in the state of Louisiana . The US did not sign the Cartagena Protocol . After lengthy approval procedures and sometimes fierce local controversies, field tests were also carried out in developing countries such as the Philippines , Bangladesh, India and Vietnam from 2008 onwards. Golden rice was crossed into local varieties; the tests necessary for approval as a cultivated plant were carried out. In some of these countries, naturally occurring color variants such as green (Vietnam) or red rice (Philippines, India) are already established.
The technology chosen for the Golden Rice represents a particularly controversial genetic engineering variant because of the transfer of alien genetic material from one organism to another. Potrykus himself had high hopes for the approach, but met with vehement opposition. Even in humanitarian projects like the Golden Rice, the production of transgenic organisms is rejected by some philosophers and ethicists as hubris , as a fundamentally wrong treatment of nature and manipulation of nature that is not due to humans. On the other hand, reference is made to the fundamentally manipulative character of agriculture such as differences in genetic engineering and the living beings modified in this way, which render such a prima facie argument ineffective. Ethical aspects of the golden rice and genetic engineering were also discussed at a meeting of the Pontifical Academy of Sciences in 2009, where Potrykus found recognition and support for his project.
Even after 2000, the development of a mass-applicable rice with sufficient vitamin contents was not as easy as it was initially shown. The first stages of development of the golden rice that were once discussed had significantly insufficient vitamin A content, and the varieties and cultivars used were not very suitable for use in developing countries. The living conditions of people in vitamin-deficient areas, extreme poverty and the low availability of fats as food also limit the intake from the rice itself. The patent law and licensing requirements and, last but not least, the financial expenditure for research and development are comparatively high. The possible income from food with genetically modified organisms is significantly lower than in the meanwhile accepted red genetic engineering , precisely because of the humanitarian focus on people in extreme poverty . Among other things, competition arose from the Golden Rice with the Harvest Plus program, which attempts to produce correspondingly more nutritious varieties through classic breeding approaches. Last but not least, the food situation, especially in Asia, has since improved independently of the rice varieties for economic reasons.
Genetic and biochemical basics
According to Peter Beyer, co-founder of the project, the genetic variability of rice is too small for conventional breeding methods to increase the β-carotene content in the rice grain itself. If the content is to be increased, genes from other, carotene-producing plants would have to be introduced. The use of genetic engineering to combat malnutrition is not restricted to transgenic varieties or varieties that have been enriched with nutrients. Other genetic engineering methods have now become mainstream in precision breeding. The use of genetic tracer processes has contributed to some of the most successful new varieties of rice in developing countries, such as the Indian variety Swarna, which is more resistant to temporary immersion. This has quickly become one of the most popular Indian rice varieties and enables significantly higher and more reliable harvest successes. Existing rice varieties are also examined using genetic engineering methods for the health effects of consumption. The Swarna mentioned is genetically considered to be less likely to cause diabetes than other conventionally bred rice varieties.
Unchanged carotene biosynthesis in rice
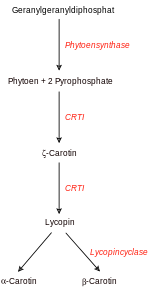
The molecule geranylgeranyl diphosphate (GGPP) is at the entrance to carotene biosynthesis . β-carotene is also called provitamin A because it is the precursor of vitamin A ( retinol ) and is converted into it in the human body.
The availability of GGPP limits all subsequent steps in the formation of carotene in the plant. Two GGPP molecules are combined by the enzyme phytoene synthase (PSY) to form one phytoene molecule . In order for phytoene to become β-carotene, it has to undergo a number of other chemical transformations. These conversions are catalyzed by specific additional enzymes . In particular, the plant enzymes phytoene desaturase (PDS), ζ-carotene desaturase (ZDS) and carotene-cis-trans-isomerase (CRTISO) must be present. The three enzymes form all- trans - lycopene from phytoene . This molecule is red in color. all- trans- lycopene will eventually be converted to β-carotene by a β-lycopene cyclase (β-LCY).
The three enzymes PDS, ZDS and CRTISO are formed in the green parts of the plant, especially the leaves, of the rice plant. There they are involved in the formation of carotene. In the rice grain itself (the endosperm ), however, the enzymes are completely or almost completely down-regulated . The yellow β-carotene is therefore not formed in the rice grain; the rice appears white.
Genetic changes in golden rice
The aim of the genetic changes was to have a coordinated set of enzymes expressed in the rice endosperm so strongly that beta-carotene is formed to a significant extent.
In order to accelerate the conversion of GGPP into phytoene, the corresponding enzyme from narcissus (NpPSY from Narcissus pseudonarcissus ) was chosen for the first golden rice . It was also known that the desaturase crtI from the bacterium Erwinia uredovora (new name: Pantoea ananatis ) can replace the three enzymes PDS, ZDS and CRTISO for converting the phytoene into all- trans- lycopene. For the further conversion of the all- trans- lycopene into beta-carotene, a β-lycopene cyclase, which comes from the daffodil (NpLCY), was initially used. The golden rice prototype described above therefore contained three alien genes ( NpPSY , NpLCY and crtI ).
The genes used were placed under the control of glutelin promoters . These promoters only work in the endosperm. The additional production of beta-carotene was thus limited to the rice grain. The prototype of the golden rice was first received in the form of the Taipei 309 variety of the Japonica type.
In golden rice plants that lacked the β-lycopene cyclase gene of the daffodil ( NpLCY ) - that is, which should not have converted the red all- trans -lycopene in the rice grain into the yellow beta-carotene - it was found that the rice grains were yellow instead of red. Further investigations showed that there is an active β-lycopene cyclase in the travel dosperm, which converts the lycopene into β-carotene at a sufficient rate. The NpLCY gene of the daffodil was therefore not necessary. As a further development of the prototype, Golden Rice 1 therefore only contains the two alien genes NpPSY and crtI .
According to further research, the phytoene synthase of the daffodil proved to be less efficient than the phytoene synthase of maize ( Zea mays ). Since this is the rate-limiting enzyme, the new variant Golden Rice 2 uses the corresponding maize gene ZmPSY instead of the gene from the daffodil.
Other applications and processes
The process developed by Beyer and Potrykus has been used in a number of other foods, staple foods as well as vegetables and fruits beyond rice. These include golden potatoes, cauliflower, kiwi fruit and golden millet , which in addition to vitamin A enrichment can also have color effects. In the meantime, further methods are being researched with which staple foods can be made more nutritious by adding transgenes. Meanwhile, varieties with increased iron or fat content are also available.
Development history and status of the project
Polished rice hardly contains any β-carotene, from which the human body can produce the vital vitamin A. Children in particular with a rice diet with little variety are therefore at risk of vitamin A deficiency. Traditional methods of plant breeding did not succeed in increasing the beta-carotene content until the mid-1980s.
Ingo Potrykus ( ETH Zurich ) and Peter Beyer ( Albert-Ludwigs-Universität Freiburg ) started developing golden rice in 1992. The first positive results ( proof of concept ) were achieved in 1999 and the first results were published in the following year.
The inventors patented the golden rice . In order to take the step from invention to product, for which Potrykus and Beyer did not receive sufficient public support, they sold the patent to Zeneca (now Syngenta ). In return, Zeneca / Syngenta provided a license for “humanitarian work” as well as technological and other support as part of a public-private partnership . As a prerequisite for the actual production of golden rice, 70 patent rights to the processes used had to be obtained from 32 patent holders. Since the Golden Rice was to be sold free of license fees, the patent holders had to agree to free use. This task was solved by the private partner in about half a year. Syngenta began developing a "commercial" line of golden rice based on the acquired patent, but later discontinued development.
In contrast to most of the other (commercially approved) properties of genetically modified plants, those of the golden rice were developed by an academic German-Swiss research group. The companies that supported the project and now also hold some patents have collaborated on this research, but foregoing financial income themselves. According to information from the manufacturer Syngenta, after crossing into local varieties, golden rice can be propagated by the farmers themselves and without a license. In July 2014, Peter Beyer stated that the patent issue had been resolved and that the seeds would be available to farmers in developing countries with an annual turnover of no more than 10,000 US dollars.
The project received financial support at this stage from the Rockefeller Foundation , the United States Agency for International Development and the Syngenta Foundation , but not from European or UN organizations.
The approval of a genetically modified plant is linked to a number of conditions and evidence of the molecular genetic structure and function of the transgenes . For example, marker genes with antibiotic resistance had to be removed and evidence of an orderly integration of the golden rice transgenes into the rice genome had to be provided. In particular, the transgenes (a) could only be present in one copy and (b) not be changed, (c) they had to be secured against unintentional expression, (d) they were not allowed to interfere with the existing expression mechanisms and (e) neither neighboring genes nor expression signals activate nor inactivate, (f) they were not allowed to activate any mobile genes present and (g) only show stable expression at the predicted rates and under the prescribed conditions. Syngenta carried out this work as part of its commercial Golden Rice project and donated the results to the humanitarian project when it ended. This work finally identified a suitable set of Golden Rice transgenes around 2003/2004.
In 2016, over 100 Nobel Prize winners called on Greenpeace to stop the campaign against the Golden Rice Project. Among other things, they argued with figures from UNICEF , which show that 2 million deaths annually are due to vitamin A deficiency.
In 2017, the authorities responsible for recognizing GMOs approved golden rice as food in Australia and New Zealand. Appropriate approvals were issued for the USA and Canada in 2018. A cultivation permit is still pending.
Field trials
In 2004, field tests began in Louisiana, USA, to test the stability of the transformation event. Golden rice seeds have been shipped to Vietnam, India and the Philippines ( International Rice Research Institute and PhilRice) for plant breeding . The deliveries were made in accordance with the provisions of the Cartagena Protocol . It took up to two years to obtain the permits, some of which were politically controversial. At the end of 2008, the first field tests were applied for in a developing country. Golden rice was crossed into local varieties; the tests necessary for approval as a cultivated plant were carried out.
The first field test in the Philippines was completed in September 2011. Further test plantings in various regions of the country are in preparation.
In January 2012, at the request of the state-owned Bangladesh Rice Research Institute (BRRI), the government of Bangladesh approved series of tests on golden rice. The decision was preceded by months of deliberations within the government.
Projected health and welfare effects
A study published in 2004 came to the conclusion that the use of golden rice in the Philippines does not completely eliminate the effects of vitamin A malnutrition (blindness, increased mortality), but can significantly improve health. Golden rice should therefore not be used as a substitute but as a complement to other programs to improve the supply of micronutrients to the population. The preliminary estimates assume that health improvements with an economic value of 16 to 88 million US dollars will occur ( DALY ). Research and development funds used here are predicted to have a return on investment of 66% to 133%.
Anderson and colleagues estimated the global welfare gains from the Golden Rice in 2005 based on a simulation at more than $ 15 billion per year. The largest amount would go to Asia. An important mechanism in this model is increased labor productivity, especially of poor Asians, through improved health.
An SEA ex ante study (2006) comes to the conclusion that a high percentage by introducing Golden Rice of mortality and morbidity caused by Vitamin A deficiency, especially in infants could be prevented, and that Golden Even under pessimistic assumptions, rice with costs of less than US $ 20 per year of life saved ( DALY ) is significantly cheaper than supplementation (US $ 134–599). In total, 40,000 lives could be saved in India every year. Under optimistic assumptions, the costs would be US $ 3 per DALY. The reason for these low costs is the problem-free reuse and distribution of the seeds from crops among farmers.
First approval was originally expected in 2002. Ingo Potrykus blames extremely high and scientifically unjustified approval hurdles for the years of delays. Wesseler / Zilberman (2014) estimate that the failure to introduce golden rice in India alone since 2002 - the year in which the variety could have been introduced at the earliest - has cost 1.42 million years of life. The authors attribute the delay, among other factors, to the political and economic power of the lobby groups, which oppose the introduction of golden rice.
Controversy
Fundamental aspects
Greenpeace doubts the effectiveness of golden rice, arguing that the funds used to develop golden rice would be better spent on existing methods to address vitamin A deficiency. This applies in particular to methods that promote organic agriculture , including food security and agricultural diversity. Also would benefit from UNICEF successfully be used to reduce the shortage of vitamin A supplements. According to Peter Beyer, a nationwide supply of vitamin A preparations is not possible in the affected countries, and this solution is by far more expensive than the vitamin A supply via golden rice. Concerning the nutritional value of golden rice, fears have been expressed that if the product is predominantly fed, an excess of provitamin A and thus vitamin A poisoning could occur. The coordinating body of the Golden Rice Project, the International Rice Research Institute , states that excess β-carotene is either stored unchanged or excreted. The institute refers to the consensus paper of an expert group on β-carotene, which in 2009 came to the conclusion that the carotenoid is a safe source for supplying the human body with vitamin A.
Another point of criticism relates to the popularity of polished rice, which was influenced by marketing activities, since unpolished rice - i.e. rice from which the aleurone layer has not been removed - has sufficient vitamin A in this layer. Peter Beyer states that the outer layers of the unpolished rice grain contain only the smallest amounts of carotenoids, which are nutritionally irrelevant.
Criticism of clinical studies
After a specialist article appeared in the American Journal of Clinical Nutrition in August 2012 on the vitamin A supply through golden rice in children, Greenpeace accused the scientists involved of endangering the test subjects because the safety of golden rice had not yet been adequately tested. The underlying study was started in 2008 in the Chinese province of Hunan. In February 2009, an international group of 22 academics criticized in an open letter published on the website of the think tank Institute of Science in Society that GR2 (Golden Rice 2) had been tested in three clinical studies on adults and children without the GR2 having gone through an official regulatory process. According to the Open Letter, the main concern of the signatories is that GR2 has never been tested on animals. There is extensive medical literature showing that retinoids, which can be formed from β-carotene, are both toxic and cause birth defects. Under these circumstances, the signatories of the open letter saw the three tests on humans as a violation of the Nuremberg Code in many ways and called on Robert Russell of the conducting Tufts University to stop the studies immediately. The head of the Golden Rice Project , Adrian Dubock, then denied that the Nuremberg Code was violated. He said the tests were allowed by independent ethics committees. It is impossible that the studies adversely affect the participants. Because humans are the designated beneficiaries of the Golden Rice, animal experiments cannot answer the questions asked.
Tufts University initiated several investigations into the study in 2012: one by Tufts' own ethics committee, another on ethics by an external team, and an internal investigation into whether there was any scientific misconduct. The examinations did not reveal any indications of a health risk for the study participants. However, the Tufts University Ethics Committee rated the conduct of the study as ethically inadequate. Insufficient evidence was found that the study had been reviewed and approved by an ethics review board in China in accordance with applicable standards. It was found that some declarations of consent were not available before the start of the experiment. There was also evidence that dates on some of the consent forms had been changed. The authors were asked to make this clear to the publishing journal. The team of external experts consulted criticized the fact that it was withheld from the study participants that the increased β-carotene in the rice tested was due to genetic modifications during the creation of the variety. In the meantime, three Chinese researchers involved in the project have been released from their positions. You are accused of violating regulations, ethical rules, and scientific integrity. The American Journal of Clinical Nutrition announced on July 29, 2015 that it was withdrawing the publication of the study results. There was no evidence of approval by a Chinese ethics committee or the full formal consent of the patients for the experiment.
literature
- G. Tang, J. Qin et al .: Golden Rice is an effective source of vitamin A. In: American Journal of Clinical Nutrition. 89, 2009, p. 1776, doi: 10.3945 / ajcn.2008.27119 . PMC 2682994 (free full text)
- AA Moghissi, S. Pei, Y. Liu: Golden rice: scientific, regulatory and public information processes of a genetically modified organism. In: Critical reviews in biotechnology. [Electronic publication before printing] July 2015, doi: 10.3109 / 07388551.2014.993586 . PMID 25603722 .
Television reports
- Daniel Mennig: The miracle rice - How a Swiss genetic engineering invention is supposed to save millions of children in SRF on March 28, 2013, 20:05, WH in 3sat on September 25, 2013 21:05
- Controversy about the film Der Wunderreis (violation of journalistic due diligence) from May 13, 2013
Web links
- Golden Rice Project self-presentation
- The history of the debate about golden rice and genetically modified wheat using the example of ETH Zurich - article on ETHistory
- The yellow revolution. (PDF; 862 kB). In: Der Spiegel . 48/2008.
- Ingo Potrykus: Lessons from the 'Humanitarian Golden Rice' project: regulation prevents development of public good genetically engineered crop products. (PDF; 216 kB). In: New Biotechnology. Volume 27, No. 5, November 2010.
- Last salvation for the golden rice. In: Spiegel Online. June 23, 2012.
- Max Rauner: Genetic engineering: Are you also ... against GM food? In: The time. July 18, 2017. (online)
Individual evidence
- ↑ a b X. Ye, S. Al-Babili, A. Klöti, J. Zhang, P. Lucca, P. Beyer, I. Potrykus: Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. In: Science . Volume 287, Number 5451, January 2000, pp. 303-305. PMID 10634784 .
- ^ Tough Lessons From Golden Rice . In: Science . tape 320 , no. 5875 , April 25, 2008, p. 468-471 , doi : 10.1126 / science.320.5875.468 , PMID 18436769 .
- ↑ a b c d e f The politics of Golden Rice . In: GM Crops & Food . tape 5 , no. 3 , July 3, 2014, p. 210-222 , doi : 10.4161 / 21645698.2014.967570 , PMID 25437240 .
- ^ Patents for Humanity Awards 2015. United States Patent and Trademark Office, accessed September 17, 2017 .
- ^ Patents for Humanity Awards Ceremony at the White House. In: IP Watchdog Blog. April 20, 2015. Retrieved September 17, 2017 .
- ↑ Penny Van Esterik: Rice and milk in Thai Buddhism: symbolic and social values of basic food substances. (PDF). In: Crossroads: An Interdisciplinary Journal of Southeast Asian Studies. Vol. 2, No. 1, 1984, pp. 46–58, Northern Illinois University Center for Southeast Asian Studies, p. 47. (full text)
- ↑ a b c d Murray W. Nabors : Botany . Addison-Wesley Verlag, 2007, ISBN 978-3-8273-7231-4 , pp. 367 ff .
- ^ Rüdinger Hahn: Multinational Companies and the "Base of the Pyramid" - New Perspectives on Corporate Citizenship and Sustainable Development. Gabler Verlag, Wiesbaden 2009, ISBN 978-3-8349-1643-3 , p. 205 (partly available from Google Books )
- ↑ Friedrich Glauner: Elements of a food ethics. In: Christoph Schank, Kristin Vorbohle (ed.): Perspective on food ethics . Rainer Hampp Verlag, Munich / Mering 2014, ISBN 978-3-95710-023-8 , p. 70.
- ↑ Dirk Zimmermann: With proven methods against vitamin A deficiency. "Golden" rice - a dangerous illusion. on Greenpeace.de, January 14, 2014.
- ↑ WHO: Micronutrient deficiencies Vitamin A deficiency , 2015.
- ↑ a b The contribution of transgenic plants to better health through improved nutrition: opportunities and constraints . In: Genes & Nutrition . tape 8 , no. 1 , August 29, 2012, p. 29–41 , doi : 10.1007 / s12263-012-0315-5 , PMID 22926437 , PMC 3534993 (free full text).
- ↑ a b Erika Strehl: Strategies for Mitigating Vitamin A Deficiency in Mekelle, Ethiopi . In: Graduate Student Research Conference. Paper 4 . University of Montana, 2015 ( umt.edu [accessed May 21, 2015]).
- ↑ Huan Lou: Golden Rice War in the Philippines: A Ban on Golden Rice Research Is Not a Wise Move following the Judicial Ban on Bt Eggplant Field-Testing. In: Minnesota Journal of International Law. 24, 2015, p. 101.
- ↑ Roukayatou Zimmermann, Matin Qaim: Potential health benefits of Golden Rice: a Philippine case study. In: Food Policy. (29), 2004, pp. 147-168.
- ↑ a b Reaz Ahmad: Vitamin-A rich rice gets nod. Govt allows trial production in Bangladesh. In: The Daily Star. February 5, 2012, accessed February 1, 2013 .
- ↑ I. Potrykus: Lessons from the 'Humanitarian Golden Rice' project: regulation prevents development of public good genetically engineered crop products. In: New biotechnology. Volume 27, Number 5, November 2010, pp. 466-472. doi: 10.1016 / j.nbt.2010.07.012 . PMID 20650337 .
- ↑ Ronald D. Sandler, Philip Cafaro: Environmental Virtue Ethics. Rowman & Littlefield, 2005, pp. 224-228.
- ^ GM Crops, the Hubris Argument and the Nature of Agriculture . Study of the hubris argument in Sandler and others. In: Journal of Agricultural and Environmental Ethics . tape 28 , no. 1 , November 15, 2014, p. 161-177 , doi : 10.1007 / s10806-014-9526-7 .
- ^ Andy Coghlan: Vatican scientists urge support for engineered crops. In: NewScientist. Retrieved May 22, 2015 .
- ^ Transgenic Plants for Food Security in the Context of Development. (PDF) In: www.ask-force.org. Retrieved May 22, 2015 (PAS Study Week appeal at the Pontifical Academy of Sciences, Casina Pio IV, Vatican May 15-19, 2009).
- ↑ Peter Beyer: Golden Rice and 'Golden' crops for human nutrition. In: New Biotechnology. 27, No. 5, 2010, pp. 478-481.
- ↑ Indian rice Swarna among most healthy varieties globally. Retrieved May 20, 2015 .
- ↑ JA Paine, CA Shipton et al .: Improving the nutritional value of Golden Rice through increased pro-vitamin A content. In: Nature Biotechnology . Volume 23, Number 4, April 2005, pp. 482-487. doi: 10.1038 / nbt1082 . PMID 15793573 .
- ↑ S. Al-Babili, P. Beyer: Golden Rice-five years on the road-five years to go? (PDF; 313 kB). In: Trends in plant science. Volume 10, Number 12, December 2005, pp. 565-573. doi: 10.1016 / j.tplants.2005.10.006 . PMID 16297656 . (Review).
- ↑ A golden era - pro-vitamin enhancement in various crops . In: In Vitro Cellular & Developmental Biology - Plant . tape 47 , no. 2 , April 14, 2011, p. 205-221 , doi : 10.1007 / s11627-011-9363-6 .
- ↑ a b c d e I. Potrykus: Lessons from the 'Humanitarian Golden Rice' project: regulation prevents development of public good genetically engineered crop products. In: New biotechnology. Volume 27, Number 5, November 2010, pp. 466-472. doi: 10.1016 / j.nbt.2010.07.012 . PMID 20650337 .
- ↑ syngenta.com (PDF)
- ↑ a b c Jost Maurin: Cell biologist on "Golden Rice": "Rice is cheaper than tablets" . In: the daily newspaper . July 27, 2014 ( taz.de ).
- ^ Laureates Letter Supporting Precision Agriculture (GMOs) - Support Precision Agriculture. In: supportprecisionagriculture.org. Retrieved January 7, 2018 .
- ^ Joel Achenbach: 107 Nobel laureates sign letter blasting Greenpeace over GMOs. In: washingtonpost.com. June 30, 2016, accessed January 7, 2018 .
- ↑ ISAAA GM Approval Database: Provitamin A Biofortified Rice. Retrieved June 13, 2018 .
- ↑ Golden Rice to hit market by 2011. ( Memento from December 3, 2013 in the Internet Archive ) on: fnbnews.com , September 1, 2009. (English)
- ↑ philrice.gov.ph, accessed February 1, 2013.
- ^ R. Zimmermann, M. Qaim: Potential health benefits of Golden Rice: a Philippine case study. (PDF; 241 kB). In: Food Policy. Volume 29, 2004, pp. 147-168.
- ↑ K. Anderson, LA Jackson, CP Nielsen: GM rice adoption: impact for welfare and poverty alleviation. In: Journal of Economic Integration. Volume 20, 2005, pp. 125-134.
- ^ Matin Qaim : The Economics of Genetically Modified Crops. ( Memento of November 5, 2013 in the Internet Archive ) (PDF; 752 kB). In: Annual Review of Resource Economics. Volume 1, 2009, pp. 665-694.
- ^ AJ Stein, HP Sachdev, M. Qaim: Potential impact and cost-effectiveness of Golden Rice. In: Nature Biotechnology . Volume 24, Number 10, October 2006, pp. 1200-1201. doi: 10.1038 / nbt1006-1200b . PMID 17033649 .
- ^ Justus Wesseler, David Zilberman: The economic power of the Golden Rice opposition. In: Environment and Development Economics. April 2014, p. 15. doi: 10.1017 / S1355770X1300065X .
- ↑ "Golden" rice - a dangerous illusion. In: greenpeace.de. January 14, 2014, accessed August 30, 2015 .
- ↑ Amy King, Mario Rautner, Glen Tyler: Golden rice's lack of luster - Addressing vitamin A deficiency without genetic engineering. (PDF; 1.4 MB) Greenpeace International, 2010, accessed on November 7, 2011 .
- ↑ Can you overdose on vitamin A from eating a lot of Golden Rice? ( Memento of March 4, 2016 in the Internet Archive ) IRRI.
- ↑ Guangwen Tang, Yuming Hu, Shi-an Yin, Yin Wang, Gerard E Dallal, Michael A Grusak, Robert M Russell: β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. (PDF; 543 kB). In: American Journal of Clinical Nutrition. 96, 2012, pp. 658-664.
- ↑ Arthur Caplan: Greenpeace out to sea on GM rice issue, bioethicist says. In: NBC News. September 14, 2012.
- ↑ Open letter from the scientists
- ↑ Sean Poulter: British scientists condemn using children in GM food trials as unacceptable In: Daily Mail . February 17, 2009.
- ↑ a b c d Martin Enserink: Golden Rice Not So Golden for Tufts. In: Science News. 18th September 2013.
- ↑ Golden rice trial triggers sackings, investigation. on: scidev.net , January 7, 2013, accessed February 2, 2013.
- ↑ Retraction of Tang G, Hu Y, Yin Sa, Wang Y, Dallal GE, Grusak MA, and Russell RM. β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am J Clin Nutr 96, 2012, pp. 658-64. In: American Journal of Clinical Nutrition. 102, 2015, p. 715, doi: 10.3945 / ajcn.114.093229 .
- ↑ 3sat program preview