Pancreatic lipase
| Pancreatic lipase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 449 amino acids | |
| Cofactor | Colipase | |
| Identifier | ||
| Gene name | PNLIP | |
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 3.1.1.3 , lipases | |
| Response type | Hydrolysis (hydrolytic ester cleavage) | |
| Substrate | Tri- / diacylglycerol + H 2 O | |
| Products | Di- / monoacylglycerin + fatty acid | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 5406 | 69060 |
| Ensemble | ENSP00000358223 | |
| UniProt | P16233 | Q6P8U6 |
| Refseq (mRNA) | NM_000936 | NM_026925 |
| Refseq (protein) | NP_000927 | NP_081201 |
| Gene locus | Chr 10: 116.55 - 116.57 Mb | Chr 19: 58.67 - 58.68 Mb |
| PubMed search | 5406 |
69060
|
The pancreatic lipase (lipase, PL) is one of two enzymes , in the small intestine of mammals recorded by the fats ( triglycerides splits). This reaction is essential for fat digestion ; around 80 percent of the triglycerides from food are already broken down by the time they reach the middle duodenum. The PL belongs to the group of lipases and is produced in the pancreas (pancreas). The cofactor protein colipase is required for this function.
The pancreatic lipase is also responsible for the hydrolysis of retinyl esters to retinol and fatty acids , which enables part of the vitamin A absorption through food (vitamin A is also supplied and absorbed directly as provitamin or as retinol).
The pancreatic lipase is the target in the drug control of obesity. The PL inhibitor orlistat has been marketed with this indication since 1998 .
Applications in medicine
Lipase is of central importance in enzyme replacement therapy in the case of impaired function of the pancreas ( pancreatic insufficiency ). In the case of cystic fibrosis in particular , the administration of enzyme preparations with pancreas powder from pigs, which are standardized to a certain lipase content, is the standard therapy. These drugs are offered with enteric coatings to protect the enzymes they contain. In some cases, lipase from non-animal sources (rhizolipase from the mold Rhizopus oryzae, trade name: Nortase ) is used, which is characterized by its natural stability against human gastric acid .
Laboratory diagnostics
In laboratory diagnostics, the activity of the lipase from heparin plasma or blood serum is measured in the clarification of upper abdominal pain, especially for the diagnosis of acute pancreatitis .
Reference range for measurements at 37 ° C (color test): serum, plasma <60 U / l
In acute pancreatitis , the lipase increases and is already above the reference range of 60 U / l 5 hours after the onset of pain. In most cases the value rises above 180 U / L and remains elevated for three to six days.
In general, the lipase determination method is less well standardized and more susceptible to interfering factors than pancreatic amylase . Therefore, when acute pancreatitis is suspected, pancreatic amylase is primarily determined in human medicine. Lipase as a supplement is useful if, for technical reasons, only the total amylase can be measured or if the patient was treated with plasma expanders (hydroxyethyl starch or Dextran 70). In veterinary medicine, on the other hand, in dogs and cats, the total concentration of pancreatic lipase in the serum is determined as pancreatic lipase immunoreactivity for the diagnosis of acute pancreatitis.
The lipase is filtered glomerularly in the kidney, but then not excreted, but reabsorbed and broken down. It therefore does not appear in the urine , but is nonetheless increased in renal insufficiency.
In the endoscopic examination of the pancreas ( ERCP = endoscopic retrograde cholangiopancreatography), the lipase increases immediately, reaches values of up to 720 U / l after six hours and remains above the reference range of 60 U / l for up to three days.
Another cause of the increase in lipase without disease value can be Gullo syndrome .
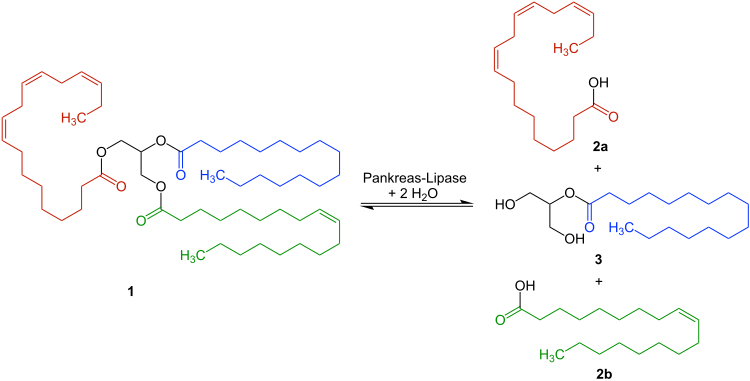
Catalyzed reaction
The pancreatic lipase cleaves triglycerides - e.g. B. 1 - only the α-fatty acid residues from. The free fatty acids 2a and 2b as well as the monoglyceride 3 are formed :
Reaction mechanism
In its active center, the lipase has a catalytic triad consisting of the amino acids aspartic acid , histidine and serine . The aspartic acid removes a proton from the histidine and thus activates it. The catalytically active histidine in turn removes a proton from the serine , which increases the nucleophilicity of the serine residue. This can now attack the carbonyl carbon of a substrate ester that is already located in the active center. A tetrahedral intermediate product forms from which an acyl- enzyme complex is formed. Deacetylation in a hydrolysis step releases the product fatty acid and the original enzyme .
See also
literature
- Birgid Neumeister, Ingo Besenthal, Hartmut Liebich (eds.): Clinical guidelines for laboratory diagnostics. 3. Edition. Urban & Fischer, Munich et al. 2003, ISBN 3-437-22231-7 .
- Lothar Thomas (ed.): Laboratory and diagnosis. Indication and evaluation of laboratory results for medical diagnostics. 6th edition. TH-Books, Frankfurt am Main 2005, ISBN 3-9805215-5-9 .
Web links
- Lipase on med4you.at
- Stability in blood samples published by the WHO (pdf) (292 kB)
- D'Eustachio / reactome.org: Digestion of triacylglycerols by extracellular PTL: colipase
- D'Eustachio / reactome.org: Digestion of diacylglycerols by extracellular PTL: colipase
Individual evidence
- ↑ UniProt P16233
- ↑ AM van Bennekum, EA Fisher, WS Blaner, EH Harrison: Hydrolysis of retinyl esters by pancreatic triglyceride lipase . In: Biochemistry . Vol. 39, No. 16, April 2000, pp. 4900-4906. PMID 10769148 .
- ↑ Ross C. Smith, James Southwell-Keely, Douglas Chesher: Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? . In: ANZ Journal of Surgery . Vol. 75, No. 6, June 2005, pp. 399-404. doi : 10.1111 / j.1445-2197.2005.03391.x . PMID 15943725 .
- ↑ Jörg M. Steiner (Ed.): Small Animal Gastroenterology. Schlütersche, Hannover 2008, ISBN 978-3-89993-027-6 .
- ^ Otto-Albrecht Neumüller (editor): Römpps Chemie Lexikon , Frank'sche Verlagshandlung, Stuttgart, 1983, 8th edition, p. 2377, ISBN 3-440-04513-7 .
- ↑ Peter Nuhn: Naturstoffchemie , S. Hirzel Wissenschaftliche Verlagsgesellschaft, Stuttgart, 2nd edition, 1990, pp. 308-309, ISBN 3-7776-0473-9 .