Aspartic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structure of L -aspartic acid, the naturally occurring enantiomer | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Aspartic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 7 NO 4 | |||||||||||||||||||||
| Brief description |
colorless leaflets or rods |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 133.10 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
269–271 ° C (decomposition) |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
poor in water (4 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Aspartic acid , abbreviated Asp or D , is one of the proteinogenic α - amino acids in its natural L - form .
Like the other amino acids, aspartic acid is normally zwitterionic in the body . In addition, however, the second carboxy group is deprotonated , which is why the term L - aspartate is often used in biochemistry instead of L -aspartic acid .
Enantiomers
Aspartic acid has a stereocenter, so there are two chiral enantiomers . In this article, the information on the physiology concerns only L -aspartic acid [synonym: ( S ) -aspartic acid]. When "aspartic acid" is spoken of without any additional name ( descriptor ), then L -aspartic acid is generally meant. The racemic DL -aspartic acid [synonym: ( RS ) -aspartic acid] and the enantiomerically pure D -aspartic acid [synonym: ( R ) -aspartic acid] are synthetically accessible and are of little practical importance.
The partial racemization of L -amino acids can be used for amino acid dating - an age determination of fossil bone material.
| Enantiomers of aspartic acid | ||
| Surname | L -aspartic acid | D -aspartic acid |
| other names | ( S ) -aspartic acid | ( R ) -aspartic acid |
| Structural formula |  |
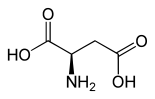
|
| CAS number | 56-84-8 | 1783-96-6 |
| 617-45-8 (racemate) | ||
| EC number | 200-291-6 | 217-234-6 |
| 210-513-3 (racemate) | ||
| ECHA info card | 100,000,265 | 100,015,668 |
| 100.009.559 (racemate) | ||
| PubChem | 5960 | 83887 |
| 424 (racemate) | ||
| DrugBank | DB00128 | DB02655 |
| - (racemate) | ||
| Wikidata | Q178450 | Q27076990 |
| Q27109481 (racemate) | ||
history
The French pharmacist Auguste-Arthur Plisson isolated asparagine from the yew tree in 1827 and proved that the “Althein” obtained from the yew tree by Pierre-Jean Robiquet is identical to the asparagine obtained from Louis-Nicolas Vauquelin and Robiquet. Plisson succeeded in synthesizing aspartic acid by reacting asparagine with lead oxide hydrate and then separating it with hydrogen sulfide (“hydrothionic acid”). He called the acid obtained "acid aspartique" (in German 'aspartic acid'). Hermann Kolbe clarified the structure of asparagine and aspartic acid in 1862.
Occurrence
L -aspartic acid was first obtained synthetically from asparagine , which occurs in the seedlings of legumes .
Vegetable asparagus ( Asparagus officinalis ) also contains quite a high proportion of this amino acid. It occurs bound in almost all proteins , in different proportions.
For the following foods rich in L -aspartic acid, the information is based on 100 g of the food, and the percentage of aspartic acid in the total protein is also given.
| Food | protein | Aspartic acid | proportion of |
|---|---|---|---|
| Soy protein , isolate | 80.69 g | 10.20 g | 12.6% |
| Egg white , dried | 81.10 g | 8.25 g | 10.2% |
| Cod , Atlantic, dried and salted | 62.82 g | 6.43 g | 10.2% |
| Peanut flour , defatted | 52.20 g | 6.37 g | 12.2% |
| Spirulina , dried | 57.47 g | 5.79 g | 10.1% |
| Tofu , freeze-dried ( Kōya-dōfu ) | 47.94 g | 5.30 g | 11.1% |
| Sunflower seed flour , partially defatted | 48.06 g | 5.16 g | 10.7% |
properties
Aspartic acid is acidic due to its two carboxy groups. This is why this amino acid is physiologically - depending on the pH - usually present as an inner salt in the form of an aspartate. Isoelectric point 2.77.
Biosynthesis and industrial production
The biosynthesis of L -aspartic acid takes place, for example, from the homologous keto acid oxaloacetate by transamination . Industrially, L -aspartic acid is obtained enantioselectively using a biotechnological process by adding ammonia to the C = C double bond of fumaric acid. A microorganism with the enzyme L -aspartase is used.
Functions
L -aspartate, the conjugate base of aspartic acid, is said to act as a transmitter in vertebrates together with glutamic acid in more than 50 percent of all synapses of the central nervous system , including the climbing fibers of the cerebellum and the moss fibers of the ammonium horn formation . It works by stimulating the NMDA receptors . However, the effect is not as strong as with glutamate .
In addition, L -aspartic acid is combined in the urea cycle by the enzyme argininosuccinate synthetase with citrulline , splitting ATP into AMP and PP i into argininosuccinate . This is then broken down into L - arginine and fumarate by the argininosuccinate lyase . L- arginine then releases urea , while fumarate is converted again in the citric acid cycle to oxaloacetate , which can be transaminated again to L -aspartate ( amino group transfer from α-amino acids to urea via transamination of oxaloacetate).
use
Significant amounts of L -aspartic acid are used in the manufacture of the sweetener aspartame . Further, L -aspartic acid as the starting material for the stereoselective synthesis of a variety of other chiral used organic chemical compounds. N- substituted polyaspartic acid esters are used as reactive components in modern paint systems.
Continues to find L aspartic application as a component of infusion solutions for parenteral nutrition and as salt formers .
See also
Web links
Individual evidence
- ↑ a b c d Entry on l-aspartic acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b c Entry on L -aspartic acid in the GESTIS substance database of the IFA , accessed on December 19, 2019(JavaScript required) .
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, 62, 1982, ISBN 3-527-25892-2 .
- ↑ A. Plisson: Sur du l`ldentité Malate acidic d`altheine avec lÀsparagine , Ann Chim Phys, Vol 36, pp 175ff (1827).
- ↑ H. Kolbe: About the chemical constitution of asparagine and aspartic acid , Liebigs Ann Chem, Volume 121, p. 232ff (1862).
- ↑ nutrient database of the US Department of Agriculture , 26th edition.
- ↑ Yoshiharu Izumi, Ichiro Chibata and Tamio Itoh: Production and Use of Amino Acids , Angewandte Chemie 90 (1978) 187-194, doi: 10.1002 / anie.19780900307 ; Angewandte Chemie International Edition in English 17 , 176–183, doi: 10.1002 / anie.197801761 .
- ↑ Philip E. Chen, Matthew T. Geballe, Phillip J. Stansfeld, Alexander R. Johnston, Hongjie Yuan, Amanda L. Jacob, James P. Snyder, Stephen F. Traynelis, and David JA Wyllie. 2005. Structural Features of the Glutamate Binding Site in Recombinant NR1 / NR2A N-Methyl-D-aspartate Receptors Determined by Site-Directed Mutagenesis and Molecular Modeling . Molecular Pharmacology . Issue 67, pp. 1470–1484.
- ^ Gary M. Coppola and Herbert F. Schuster: Asymmetric Synthesis - Construction of Chiral Molecules Using Amino Acids , Wiley, 1987, pp. 204-213, ISBN 0-471-82874-2 .
- ^ S. Ebel and HJ Roth (editors): Lexikon der Pharmazie , Georg Thieme Verlag, 1987, p. 66, ISBN 3-13-672201-9 .
