Selenomethionine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula without specifying the stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Selenomethionine | ||||||||||||||||||
| other names |
2-amino-4- (methylselenyl) butyric acid |
||||||||||||||||||
| Molecular formula | C 5 H 11 NO 2 Se | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 196.1 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
265-267 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Selenomethionine is an α - amino acid and an analogue of the amino acid methionine .
Stereochemistry
Selenomethionine has a stereocenter, so there are two enantiomers . If the name 'selenomethionine' is not identified by a descriptor , it means L- selenomethionine [synonym: ( S ) -selenomethionine].
D -Selenomethionine [Synonym: ( R ) -Selenomethionine] is the enantiomer of L- Selenomethionine and does not occur in nature.
| Enantiomers of selenomethionine | ||
| Surname | L -elenomethionine | D -elenomethionine |
| other names | ( S ) -elenomethionine | ( R ) -selenomethionine |
| Structural formula |
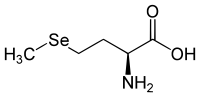
|
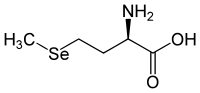
|
| CAS number | 3211-76-5 | 13091-98-0 |
| 1464-42-2 (unspec.) | ||
| EC number | 608-705-0 | - |
| 215-977-0 (unspec.) | ||
| ECHA info card | 100.123.183 | - |
| 100.014.525 (unspec.) | ||
| PubChem | - | 5460538 |
| 15103 (unspec.) | ||
| DrugBank | DB11142 | - |
| - (unspec.) | ||
| Wikidata | Q27096144 | Q27110364 |
| Q415925 (unspec.) | ||
properties
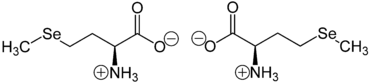
Selenomethionine is mainly present as an "inner salt" or zwitterion , the formation of which can be explained by the fact that the proton of the carboxy group migrates to the lone pair of electrons on the nitrogen atom of the amino group :
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value), at which the selenomethionine also has its lowest solubility in water. Selenomethionine is not one of the essential amino acids.
synthesis
The synthesis of racemic selenomethionine starts from α-bromo-γ-butyrolactone. The halogen is substituted for an amino group by reaction with ammonia . The resulting hydrogen bromide is neutralized with barium hydroxide . When acidifying with sulfuric acid , the hydrobromide of α-amino-γ-butyrolactone is formed. Its ring opening with potassium acetate and ethanol yields a diketopiperazine , which is also a diol with two primary hydroxyl groups. The nucleophilic substitution with potassium methyl selenide and subsequent acidic work-up then yields ( RS ) -selenomethionine.
use
L -elenomethionine is used in the X-ray structure analysis of proteins. During the recombinant protein synthesis, selenomethionine is built into the proteins instead of methionine. This is used to solve the phase problem ( Patterson method ).
L -elenomethionine is also used to supply humans with the trace element selenium: this amino acid is fed to yeasts for this purpose , and the yeasts in turn are processed into pharmaceutical preparations.
Others
- Abbreviation: SeMet, Sem
- Residual name: selenomethionyl
- Side chain : lipophilic
See also
Individual evidence
- ↑ a b c data sheet Seleno-L-methionine from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ^ A b X. G. Ran, DR Cao, LY Wang, YC Lin: A Convenient Synthesis of D, L-Selenomethionine , Polish J. Chem. 83 ( 2009 ) 431-435.
- ↑ Michael Pieper, Michael Betz, Nediljko Budisa , Franz-Xaver Gomis-Rüth, Wolfram Bode, Harald Tschesche: Expression, Purification, Characterization, and X-Ray Analysis of Selenomethionine 215 Variant of Leukocyte Collagenase , in: J Protein Chem. , 1997 , 16 (6) , pp. 637-650; PMID 9263126 ; doi: 10.1023 / A: 1026327125333 .



![Synthesis of (RS) -selenomethionine. [2]](https://upload.wikimedia.org/wikipedia/commons/thumb/8/82/Selenomethionine_synthesis_v.2.png/480px-Selenomethionine_synthesis_v.2.png)