Tryptophan
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Illustration of L- Triptophan, the naturally occurring form | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tryptophan | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 11 H 12 N 2 O 2 | |||||||||||||||||||||
| Brief description |
white to beige solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
|
|||||||||||||||||||||
| Mechanism of action |
Pre-hormone, food |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 204.23 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| boiling point |
281–282 ° C (sublimation at 0.4 hPa) |
|||||||||||||||||||||
| Vapor pressure |
28 µ Pa at 25 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tryptophan , abbreviated as Trp or W , is in the L form (see Fischer projection ) a proteinogenic α - amino acid with an aromatic indole ring system. Together with phenylalanine , tyrosine and histidine, tryptophan is therefore one of the aromatic amino acids. It is one of the essential amino acids , so it cannot be produced by the human body and must be taken in with food.
Isomers
Tryptophan has a stereocenter, so there are two enantiomers. The naturally occurring form is called L -Tryptophan [Synonym: ( S ) -Tryptophan]. The enantiomer D- tryptophan (mirror image of L- tryptophan) and the racemate (1: 1 mixture of D- and L-form) are of little importance. If “tryptophan” is mentioned in this article or in the literature without any additional name ( prefix ), L- tryptophan is meant.
| Isomers of tryptophan | ||
| Surname | L -Tryptophan | D -Tryptophan |
| other names | ( S ) -Tryptophan | ( R ) -Tryptophan |
| Structural formula | 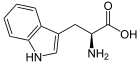 |

|
| CAS number | 73-22-3 | 153-94-6 |
| 54-12-6 (unspec.) | ||
| EC number | 200-795-6 | 205-819-9 |
| 200-194-9 (unspec.) | ||
| ECHA info card | 100,000,723 | 100.005.292 |
| 100,000,178 (unspec.) | ||
| PubChem | 6305 | 9060 |
| 1148 (unspec.) | ||
| DrugBank | DB00150 | DB03225 |
| - (unspec.) | ||
| Wikidata | Q181003 | Q27077125 |
| Q27103394 (unspec.) | ||
Occurrence
Tryptophan is a component of proteins and peptides . Since the human organism is unable to produce this amino acid, it is dependent on it to be supplied with food. The following examples each relate to 100 g of the food; the percentage of tryptophan in the total protein is also given:
| Food | protein | Tryptophan | proportion of |
|---|---|---|---|
| Soybeans | 36.49 g | 590 mg | 1.6% |
| Cashew nuts | 18.22 g | 287 mg | 1.6% |
| Cocoa powder, unsweetened | 19.60 g | 293 mg | 1.5% |
| oatmeal | 13.15 g | 182 mg | 1.4% |
| Cow's milk, 3.7% fat | 3.28 g | 46 mg | 1.4% |
| Rice, unpeeled | 7.94 g | 101 mg | 1.3% |
| Chicken egg | 12.57 g | 167 mg | 1.3% |
| Chicken breast fillet, raw | 21.23 g | 267 mg | 1.3% |
| Peas, dried | 24.55 g | 275 mg | 1.1% |
| Walnuts | 15.23 g | 170 mg | 1.1% |
| Pork, raw | 20.95 g | 220 mg | 1.1% |
| Salmon, raw | 20.42 g | 209 mg | 1.0% |
| Wholemeal corn flour | 6.93 g | 49 mg | 0.7% |
All of these foods only contain chemically bound L- tryptophan as a protein component, but no free L- tryptophan.
Estimates of the daily requirement for healthy adults range, depending on the method used, from 3.5 to 6 mg tryptophan per kilogram of body weight. There is evidence that the need for tryptophan can vary greatly from person to person.
properties
The amino acid side chain of tryptophan is lipophilic and aromatic . It is therefore poorly soluble in water. Its isoelectric point is 5.89, the pK COOH is 2.4, the pK NH 2 9.3 (both at 25 ° C).
Tryptophan is sensitive to oxidation . It can be oxidized to 2-hydroxytryptophan under comparatively mild conditions, for example by dimethyl sulfoxide (DMSO) in hydrochloric acid .
The van der Waals volume of tryptophan is 163 and the degree of hydrophobicity is −0.9. Free tryptophan as well as protein-bound tryptophan units fluoresce under ultraviolet radiation . When excited with UV light with a wavelength of 280 nm, the fluorescence emission occurs between 308 and 350 nm, depending on the polarity of the tryptophan's direct environment. If tryptophan units are present in proteins, the fluorescence of tryptophan covers the fluorescence of the other aromatic amino acids ( tyrosine , phenylalanine ).
Extraction and presentation
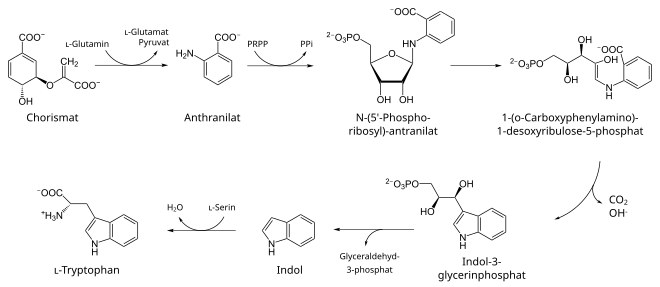
biosynthesis
Plants and microorganisms can produce L- tryptophan, among other things from shikimic acid via the shikimic acid route , whereby the enzyme anthranilate synthase ( EC 4.1.3.27 ) catalyzes the conversion of chorismate into anthranilate . The latter condenses with phosphoribosyl pyrophosphate (PRPP) with elimination of diphosphate to form N- (5-phosphoribosyl) anthranilate (by anthranilate-phosphoribosyl transferase , EC 2.4.2.18 ). After isomeric rearrangement of the ribose component (by the phosphoribosyl anthranilate isomerase , EC 5.3.1.24 ), under the influence of indole-3-glycerol phosphate synthase ( EC 4.1.1.48 ), ring closure to indole-3-glycerol phosphate follows with decarboxylation. In the last two steps indole cleaved, and then from that with L - Serine the L formed tryptophan, both optionally catalyzed by tryptophan synthase ( EC 4.2.1.20 ).
In a bacterium like E. coli , the cellular tryptophan synthesis is controlled via the trp operon , in which the segments of the genes trpE , GD , CF , B and A follow one another according to a regulatory area - here for repression and also for attenuation . These are transcribed together as polycistronic mRNA and translated into polypeptides lead to the formation of the sometimes heterotetrameric associated proteins. These multi-enzyme complexes unfold depending according to their sub-units, the effectiveness of anthranilate synthase (trp E and trp-G) and anthranilate phosphoribosyl transferase (trp-D), phosphoribosyl anthranilate isomerase (TRP-F) and indole glycerol phosphate synthase (trp-C), as well as tryptophan synthase (trp-A and trp-B).
Industrial synthesis
The industrial production of L- tryptophan is also done biosynthetically from L -serine and indole and uses a wild-type mutant of Escherichia coli . The conversion is catalyzed by the enzyme tryptophan synthase.
use
Medicines / Food Supplements / Nutrition
As a component of nutrient solutions for parenteral nutrition , L- Triptophan is widely used, along with other amino acids.
effect
The effects of L- Triptophan are often described as mood-enhancing, calming and weight-reducing. The mood-enhancing effect of L- Triptophan is probably based on the fact that it is converted to serotonin in the human body . It is believed that increased levels of serotonin can improve mood and relieve depression . The main side effects of high doses are daytime sleepiness, dizziness, drowsiness, nausea, diarrhea and headaches .
The plasma half-life is 2 ± 0.1 hours; in liver diseases such as liver cirrhosis this can increase to 4.7 ± 0.4 h.
pharmacology
L -Tryptophan is considered a "natural antidepressant ", it is said to have a certain effectiveness in depressive illnesses with at the same time low side effects. However, there is no scientific evidence of the effect of additional tryptophan administration (e.g. as a dietary supplement).
As a simple dietary supplement, L- Triptophan is only useful if there is a confirmed deficiency, which is practically unknown in industrialized countries. With fructose malabsorption , however, a significantly reduced serum tryptophan level has been observed. The level of this essential L- amino acid in the nutrient fluid of the brain cannot be adjusted at will by consuming protein- rich food, because L- tryptophan competes with five other amino acids at the blood-brain barrier for penetration into the nutrient fluid of the brain; namely with the branched chain (these are L - valine , L - leucine and L - isoleucine ) and two aromatic ( L - phenylalanine and L - tyrosine ) amino acids. Nevertheless, the L- tryptophan level can be raised through food intake by consuming carbohydrates with a protein-rich meal. Because of an increased insulin level, the branched-chain amino acids are preferentially absorbed by the muscles of the body. Although the amino acids valine, leucine, isoleucine, arginine and phenylalanine stimulate the release of insulin, the release of insulin is additionally stimulated by carbohydrates and the effect is increased. The competition for the carrier proteins at the blood-brain barrier is reduced, and tryptophan as well as phenylalanine and tyrosine can cross the blood-brain barrier more easily as a result.
In the case of serotonin deficiency, L - 5-hydroxytryptophan can be taken instead of L- tryptophan , which should be metabolized to serotonin much more efficiently, so that the dose can be reduced. However, the use is associated with the risk of serious damage to health with long-term and / or high-dose use and generally with more side effects.
dosage
An overdose of L- tryptophan is difficult because L- tryptophan itself is the main activator of its degrading enzyme tryptophan pyrrolase (more precisely: tryptophan-2,3-dioxygenase , EC 1.13.11.11 ). Another activator is cortisol . This also explains why stress (and the resulting increased cortisol levels) leads to a reduced conversion of L-tryptophan to 5-HTP. Nicotinic acid (vitamin B 3 ), on the other hand, inhibits the activity of the enzyme and thus promotes the conversion of L- tryptophan to 5-HTP. Tryptophan pyrrolase breaks down L- tryptophan with consumption of oxygen to N -formyl-L- kynurenine , which can be converted into other substances (including nicotinic acid). This is also the main metabolic pathway of L- tryptophan (only about 3% is converted to 5-HTP or serotonin). Heme (iron) acts as a cofactor. Above a certain limit, an increase in the supply of L- tryptophan is offset by a disproportionate activation of the tryptophan pyrrolase, so that as a result, more L- tryptophan is broken down than was additionally supplied. Therefore, when used therapeutically, it can make sense to take a break from taking at least once a week.
Legal situation
L -Tryptophan is not approved for the treatment of depressive illnesses in Germany. As a mild sleeping aid , tryptophan-containing drugs may be dispensed without a prescription (available as tablets with 500 mg L- tryptophan in variable pack sizes). The label on the pack must warn of consumption by pregnant and breastfeeding women as well as children and adolescents, indicate the possible impairment of the ability to drive and advise a doctor or therapist to be consulted before consumption. In Austria and Switzerland, L -Tryptophan requires a prescription.
Tryptophan scandal, 1989
L -Tryptophan was banned in the United States until January 1996. The ban goes back to the use of presumably contaminated L- Triptophan by the Japanese company Shōwa Denkō in the 1980s. The impure substance contained u. a. 'Dimer' tryptophan derivatives, produced by genetic engineering, are said to have been responsible for the occurrence of EMS cases ( eosinophilia-myalgia syndrome ), some of which were fatal. The origin of EMS is not fully understood and its recurrence in connection with the intake of tryptophan cannot be ruled out. Finally, symptoms of EMS with tryptophan derivatives and with uncontaminated tryptophan could also be triggered in animal experiments.
Feed
Many types of grain have too low an essential amino acid content. As a result of this lack of only one amino acid, the usability of all the amino acids taken in drops to the value determined by the essential amino acid (“limiting amino acid”) contained in too small a quantity; the biological value is reduced. The nutritional value of the grain can then be increased through the targeted addition of small amounts of those essential amino acids that are deficient in them. The addition of L- Triptophan to compound feed is widespread in the feed industry.
Biological importance
The codon UGG codes for the amino acid tryptophan.
- L -Tryptophan is involved in the construction of various proteins in the human body , e.g. B. in the muscles , in apolipoprotein B100 (part of the cholesterol transport molecule LDL ) or in enzymes .
- It serves as a precursor for various messenger substances ( neurotransmitters , hormones ) such as serotonin and melatonin .
- L -Tryptophan is a provitamin for vitamin B 3 .
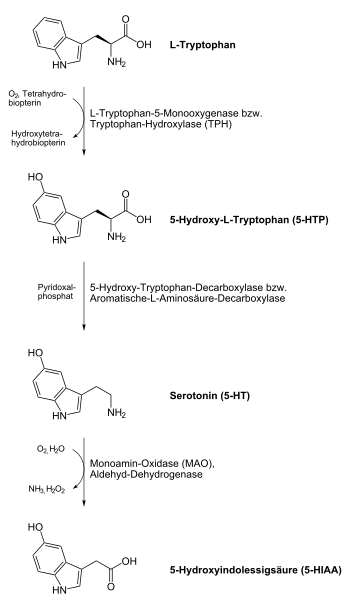
Serotonin synthesis (human)
L -Tryptophan is converted into 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase (TPH, EC 1.14.16.4 ) . Tryptophan hydroxylase can be inhibited by a number of factors, such as: As by vitamin - B 6 - / vitamin B 3 deficiency, insulin resistance , magnesium deficiency, but also by stress . Since the transition from L- tryptophan to 5-HTP is the rate-determining step in the body's own serotonin synthesis, tryptophan hydroxylase has an important regulatory function in this synthesis pathway.
5-HTP (also known as oxitriptan) is converted into serotonin by the enzyme hydroxytryptophan decarboxylase (more precisely: aromatic L-amino acid decarboxylase , AADC, EC 4.1.1.28 ) . The vitamin B 6 derivative pyridoxal phosphate acts as a cofactor and, depending on its presence, increases (or decreases) the activity of hydroxytryptophan decarboxylase.
Breakdown of tryptophan
The degradation of L- tryptophan requires the cleavage of both aromatic rings, which is catalyzed by oxygenases . The pyrrole ring is first broken by the tryptophan-2,3-dioxygenase , whereby kynurenine is produced via the splitting off of formic acid (with the help of the arylformamidase ) . This is converted to 3-hydroxykynurenine by kynurenine-3-monooxygenase ( EC 1.14.13.9 ). The cofactor is FAD , while the cosubstrates are molecular oxygen and NADPH . After alanine has been split off (using kynureninase ), another dioxygenase, 3-hydroxyanthranilate-3,4-dioxygenase , catalyzes the splitting of the remaining aromatic ring, so that after several reaction steps, acetacetate is finally present. The unstable aldehyde, which is formed after the application of the second dioxygenase and spontaneously converts to quinolinate , is partly used in NAD biosynthesis .
Several of the enzymes involved in the breakdown appear to lose activity in rats with age.
The oxidation of tryptophan or tryptophan units of proteins by photo- oxidation or oxygen radicals results in a large number of oxidation products, all of which have not yet been identified.
Tryptophan and immune system
Indolamine-2,3-dioxygenase (IDO) is an isoenzyme of tryptophan-2,3-dioxygenase (tryptophan pyrrolase), which is activated during an immune reaction to ensure the availability of tryptophan for e.g. B. virus-infected cells or cancer cells and thus limit their growth. For this reason, reduced tryptophan levels with a simultaneously increased rate of degradation are also observed in the blood of patients with such diseases: the more pronounced the tryptophan reduction in the patient, the greater the probability of a shorter survival time. The reduced availability of tryptophan is also one of the reasons for an increased tendency towards depression in these patients.
Trade names
Ardeydorm (D), Ardeytropin (D), Kalma (A, D), and a generic (D)
AKE (D), Alvesin (D), Aminofusin (D), Aminomel (D, A), Aminomix (D, A), Aminopäd (D, A), Aminoplasmal (D, A), Aminosteril (D), Aminoven ( D), Clinimix (D, A), Custodiol (D, A), Deltamin (D), Glamin (D), Glavcamin (A), Infesol (D), Intrafusin (D), Kabiven (D), Nephrotect (D ), Nutriflex (D, A), OliClinomed (D, A), Pädamin (A), Parentamin (D), Periplasmal (D, A), Salviamin (D), SmofKabiven (A), StructoKabiven (D, A), Synthamin (D), Vamin (A), Vitromix (A)
See also
literature
- Berg / Tymoczko / Stryer: Biochemistry , 5th edition, Spektrum Akademischer Verlag GmbH Heidelberg 2003, ISBN 3-8274-1303-6 .
- Burger / Wachter: Hunnius Pharmaceutical Dictionary Walter de Gruyter, 7th edition, Verlag 1993, ISBN 3-11-013868-9 .
Web links
- Independent pharmaceutical information about L-tryptophan (1989) at the Medical University of Innsbruck
Individual evidence
- ↑ a b c d data sheet tryptophan (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b c Entry on tryptophan in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c d Entry on L-tryptophan. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ Hans Beyer , Wolfgang Walter : Textbook of organic chemistry . Hirzel Verlag, Stuttgart 1991, ISBN 3-7776-0485-2 , p. 823.
- ↑ a b Data sheet DL-Tryptophan, ≥99% from Sigma-Aldrich , accessed on February 27, 2013 ( PDF ).
- ↑ Nutrient Database of the US Department of Agriculture , 22nd Edition.
- ^ VR Young, AE El-Khoury: Human amino acid requirements: A re-evaluation . In: The United Nations University Press - Food and Nutrition Bulletin 17 (3); Sept 1996 (full text) .
- ↑ G. Lazaris-Brunner, M. Rafii, RO Ball, PB Pencharz: Tryptophan requirement in young adult women as determined by indicator amino acid oxidation with L- [13C] phenylalanine. In: Am. J. Clin. Nutr. , 68 (2), Aug 1998, pp. 303-310, PMID 9701187 .
- ↑ WE Savige, A. Fontana: Oxidation of tryptophan to oxindolylalanine by dimethyl sulfoxide-hydrochloric acid. Selective modification of tryptophan containing peptides. In: International journal of peptide and protein research. Volume 15, Number 3, March 1980, pp. 285-297, PMID 6155358 .
- ↑ ER Radwanski, RL Last: Tryptophan biosynthesis and metabolism: biochemical and molecular genetics . In: Plant Cell , 1995, 7 (7), pp. 921-934, PMID 7640526 , plantcell.org (PDF).
- ↑ Charles Yanofsky : RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria . In: RNA . tape 13 , no. 8 , August 2007, p. 1141–1154 , doi : 10.1261 / rna.620507 , PMC 1924887 (free full text).
- ↑ https://www.vitaminexpress.org/de/tryptophan
- ↑ M. Rössle, R. Herz, W. Hiss, W. Gerok: The tryptophan stress test as a functional parameter in liver diseases. , In: Klinische Wochenschrift , Volume 61, Issue 6, March 1983, pp. 277-283, doi: 10.1007 / BF01497776 .
- ↑ Jana Meixner: Effect not proven: tryptophan against depression. Medicine transparent , May 25, 2020, accessed on July 7, 2020 .
- ↑ M. Ledochowski, B. Widner, D. Fuchs: Fructose malabsorption and the decrease of serum tryptophan concentration . In: G. Huether, W. Cooking, TJ Simat, H. Steinhart (Eds.): Tryptophan, serotonin, and melatonin: basic aspects and applications . Kluwer Academic / Plenum Publishers, New York 1999, pp. 73-78.
- ↑ a b L -Tryptophan - nature's answer to Prozac ( Memento from March 16, 2016 in the Internet Archive ) by James South MA.
- ↑ Announcement of a general decree in accordance with Section 54 of the Food and Feed Code (LFGB) for bringing into the Federal Republic of Germany and the placing on the market of a dietary supplement with the addition of L-tryptophan (BVL 14/01/004) dated February 24, 2014. Federal Office for Consumer Protection and food safety. Federal Gazette , March 6, 2014.
- ↑ List of substances ( memento of February 14, 2016 in the Internet Archive ) of SwissMedic; As of January 31, 2016.
- ^ Brian L. Williamson, Linda M. Benson, Andy J. Tomlinson, Arthur N. Mayeno, Gerald J. Gleich, Stephen Naylor: On-line HPLC-tandem mass spectrometry analysis of contaminants of l-tryptophan associated with the onset of the eosinophilia-myalgia syndrome . In: Toxicology Letters , 92, 1997, pp. 139-148, doi: 10.1016 / S0378-4274 (97) 00048-9 .
- ^ The Medicines Letter, year 2000, edition 3, p. 23 .
- ↑ Yoshiharu Izumi, Ichiro Chibata, Tamio Itoh: Production and Use of Amino Acids . In: Angewandte Chemie , 90, 1978, pp. 187-194. Angewandte Chemie International Edition in English , 17, pp. 176–183.
- ↑ Manfred Kircher, Wolfgang Leuchtenberger: Amino acids - a contribution to world nutrition . In: Biology in our time , 28, 1998, pp. 281-293.
- ↑ Steven B. Harris: 5-HTP: Doc Harris Presents Green Banana Award .
- ↑ S Comai, CV Costa, E Ragazzi, A Bertazzo, G Allegri: The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats . In: Clin. Chim. Acta . 360, No. 1-2, October 2005, pp. 67-80. doi : 10.1016 / j.cccn.2005.04.013 . PMID 15970278 .
- ↑ B. Widner, A. Laich, B. Sperner-Unterweger, M. Ledochowski, D. Fuchs: Neopterin production, tryptophan degradation, and mental depression - what is the link? . In: Brain Behav. Immunity . 16, 2002, pp. 590-595.